3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing
About the product
The 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing is an antimicrobial dressing that combines infection reduction, site visibility, consistent application and catheter securement in one integrated, easy-to-use product. It is the only transparent CHG dressing proven to reduce catheter-related bloodstream infections (CRBSI) and vascular catheter colonisation that aligns with evidence-based guidelines and practice standards.
Product details
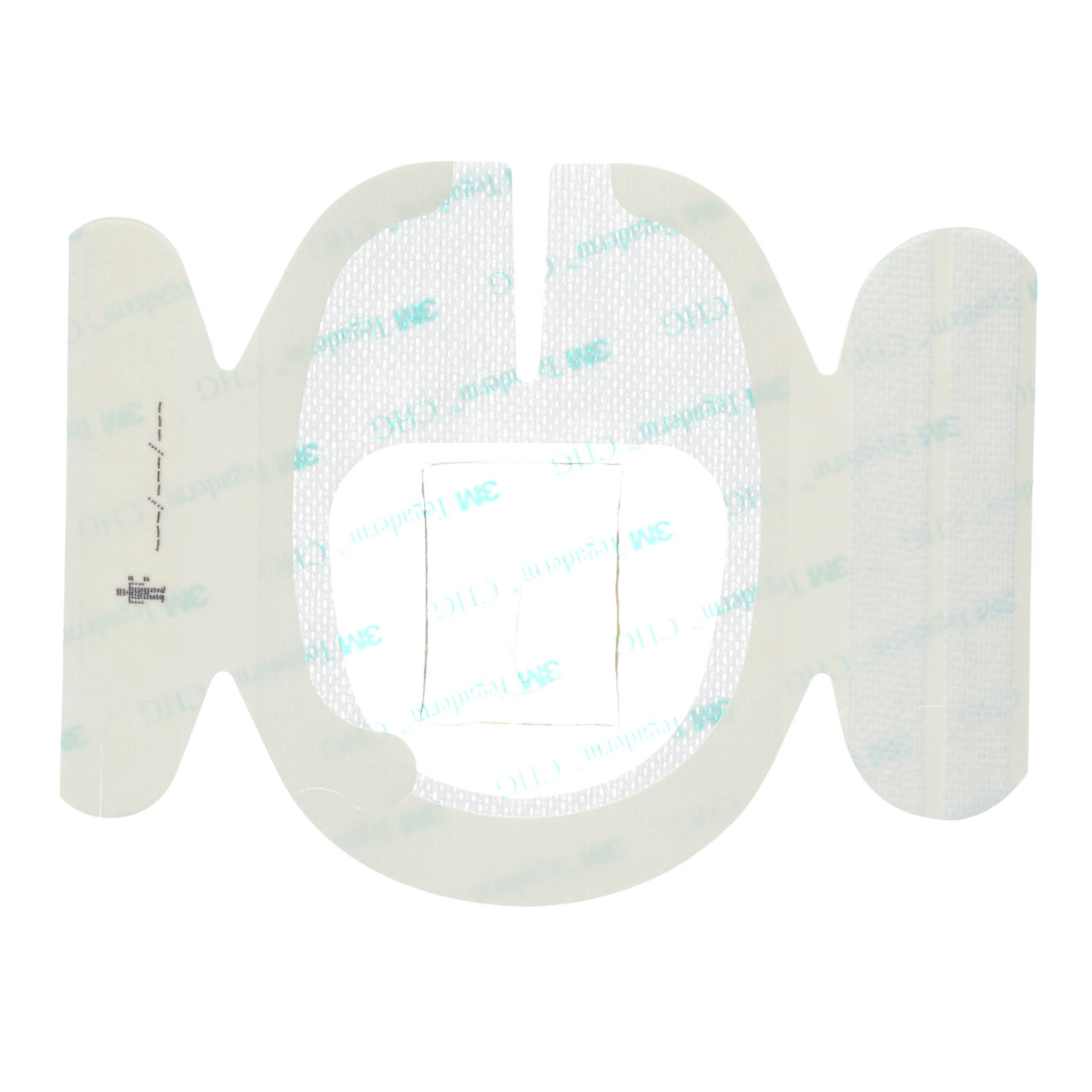
The 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing integrates a chlorhexidine gluconate (CHG) gel pad and is the only transparent dressing cleared and proven to reduce catheter-related bloodstream infections (CRBSI) and vascular catheter colonisation. It provides immediate and continuous antimicrobial protection for up to 7 days with a highly breathable transparent film that offers a waterproof, sterile barrier against external contaminants*. Both the film and the CHG gel pad remain clear for continuous site observation.
A reinforced stabilisation border provides strength needed to stabilise the catheter. A conforming keyhole notch allows catheter lumens to fit better and stay in place, and the conforming edge border uses technology designed to reduce edge-lift. A securement tape strip with a notch promotes consistent application and enhances stabilisation, and a documentation tape strip pre-printed for documenting dressing changes can provide additional securement.
*In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
Note/Important Safety Warning: Do not use 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing on premature infants or infants younger than 2 months of age. Use of this product on premature infants may result in hypersensitivity reactions or necrosis of the skin. The safety and effectiveness of 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing has not been established in children under 18 years of age. For full prescribing information, see the Instructions for Use. RX Only- Only transparent dressing cleared and proven to reduce catheter related bloodstream infections (CRBSI) and vascular catheter colonisation.
- Integrated design combines infection reduction with site visibility, catheter securement and consistent application.
- CHG gel pad provides immediate and continuous antimicrobial protection against microorganisms associated with CRBSIs for up to 7 days.
- Transparent film and gel pad allows continuous site visibility to easily assess for early signs of infection.
- Designed to minimise catheter movement and dislodgement.
- Large securement tape strip with notch promotes consistent, correct application.
- Can be worn up to 7 days.
- Provides a waterproof, sterile barrier to external contaminants including liquids, bacteria and viruses.*
- Application frame makes placement accurate and easy, minimising potential to stick to gloves or to itself.
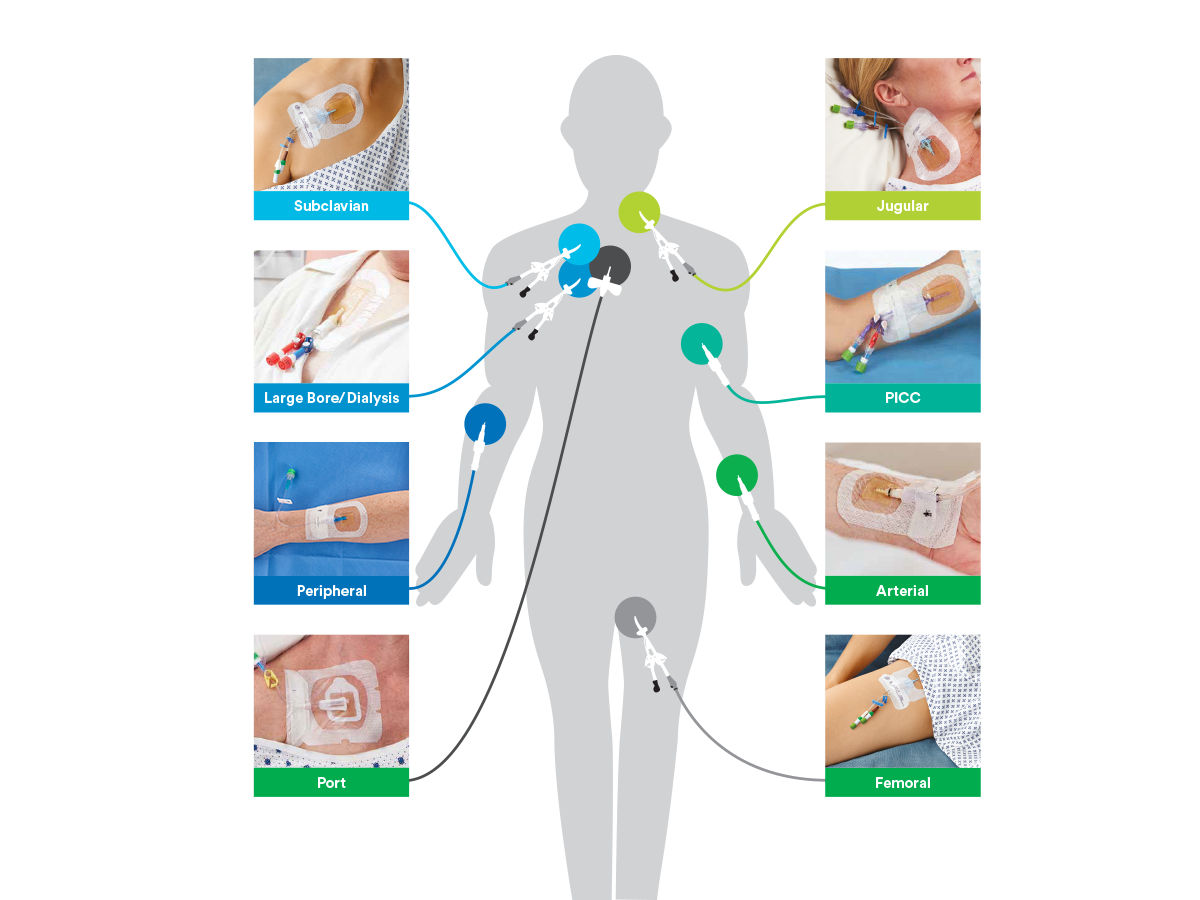
Suggested Applications
- Central venous catheters (including subclavian, jugular, femoral and PICCs)
- Dialysis catheters
- Arterial catheters
- Short peripheral I.V. and midline venous catheters
- Epidural catheters
- Other percutaneous devices
Legal
*In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.Product specifications
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
4.72 in, 6.1 in, 4.53 in, 3.35 in |
| Overall Width (Metric) |
Overall Width (Metric)
10 cm, 7 cm |
| Overall Length (Metric) |
Overall Length (Metric)
12 cm, 15.5 cm, 11.5 cm, 8.5 cm |
| Category Name |
Category Name
Antimicrobial IV Dressings |
| Dressing Type |
Dressing Type
Film |
| OverallWidthMetric |
OverallWidthMetric
10 cm, 8.5 cm |
| Overall Width (Imperial) |
Overall Width (Imperial)
3.94 in, 3.35 in, 2.76 in |
| categoryName |
categoryName
Antimicrobial IV Dressings |
There was an error processing your request. Please try again later.