3M™ Cavilon™ No Sting Barrier Film
9 Products available
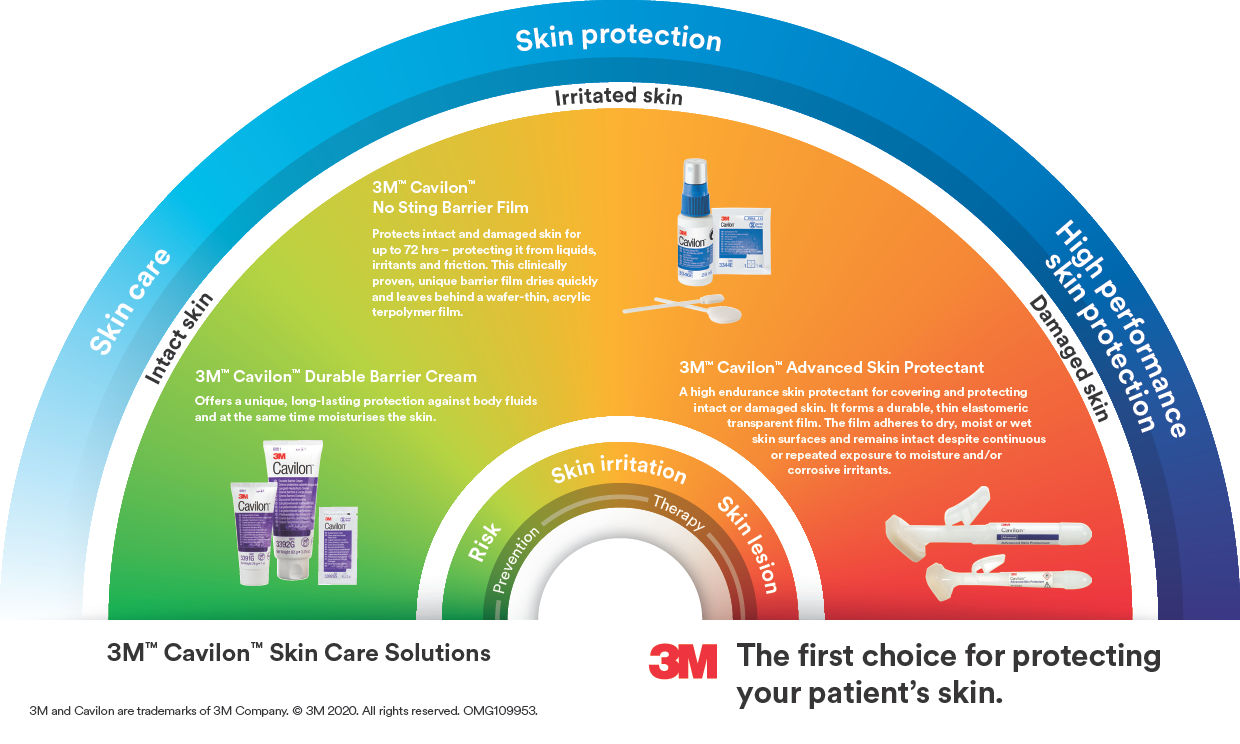
About the product
3M™ Cavilon™ No Sting Barrier Film is the original alcohol-free liquid barrier film that dries quickly to form a breathable, transparent coating on the skin.
Product details
It is designed to protect intact, damaged or 'at-risk' skin from urine, faeces, other body fluids, medical adhesive related skin injury (MARSI) and friction. It lasts for up to 72 hours¹ ² , and will not sting when applied to broken skin.
- Primary barrier against body fluids, for improved continence care that doesn’t require removal
- Alcohol-free and non-stinging, comfortable to use even on sore, damaged skin
- Safe; will not interfere with the healing process of sore, damaged skin³ ⁴
- Fast-drying and non-sticky, for ease of use and patient comfort⁵
- Proven to help protect the skin during IV site care⁶. Chlorhexidine gluconate and povidone iodine compatible⁷
- Peel-open pouch facilitates aseptic delivery technique (3343 only)
- Allows adhesives to stick
- A proven solution with over 80 pieces of evidence supporting its efficacy and cost savings⁸ ⁹
Suggested Applications
- Prevention of IAD (incontinence-associated dermatitis)⁸
- Peristomal/Peritube skin protection⁸
- Periwound skin protection⁸
- Medical adhesive-related skin injury (MARSI) prevention around I.V. sites, under dressings, tapes and negative pressure wound therapy (NPWT)⁸
- Protection from moisture and friction ¹⁰ (intertriginous dermatitis)
Legal
¹ 2010 Grove et al. 3M White Paper. A Comparison of the Durability of Four Barrier Film Products Over a 72 Hour Period on Human Volunteers. ² CLIN-RPT-FINAL-ICH3-US-05-155867 EM-05-012107 Study to Determine the Ability of the Carbon Black Retention Method to Assess the Durability of Film-Forming Agents, Sponsor Final report (2010). ³ Cameron J, Hoffman D, Wilson J, Cherry G. “Comparison of two peri-wound skin protectants in venous leg ulcers: a randomized controlled trial”. J Wound Care, vol. 14, no. 5, 2005, pp. 233-236. ⁴ Rueda Lopez J, et.al. “A comparative study of a barrier product versus zinc oxide for the treatment of incontinent lesions”. Oral presentation at the World Union Wound Healing Society (WUWHS) Meeting in Paris; 2004. ⁵ CLIN-RPT-FINAL-INV-US-05-289804 EM-05-013869 Study to Assess the Durability of Film-Forming Barriers Using the Activated Carbon Retention Method (2016). ⁶ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁷ 3M Data on file. ⁸ CLIN-SUPPORT-05-863013 NSBF Clinical Evidence Summary Brochure (2022). ⁹ CLIN-MISC-US-05-333303 Additional Clinical Evidence for NSBF (2017). ¹⁰ CLIN-MISC-US-05-169008 NSBF Friction Study Grove (2011)Product specifications
| Brand |
Brand
Cavilon™ |
| Volume (Imperial) |
Volume (Imperial)
0.034 fl oz (us), 0.101 fl oz (us), 0.947 fl oz (us) |
| Category Name |
Category Name
No-Sting Barrier Films |
| Volume (Metric) |
Volume (Metric)
1 ml, 3 ml, 28 ml |
| Delivery System |
Delivery System
Wand, Spray, Wipe |
There was an error processing your request. Please try again later.