3M™ Tegaderm™ Antimicrobial I.V. Advanced Securement Dressing 9132
About the product
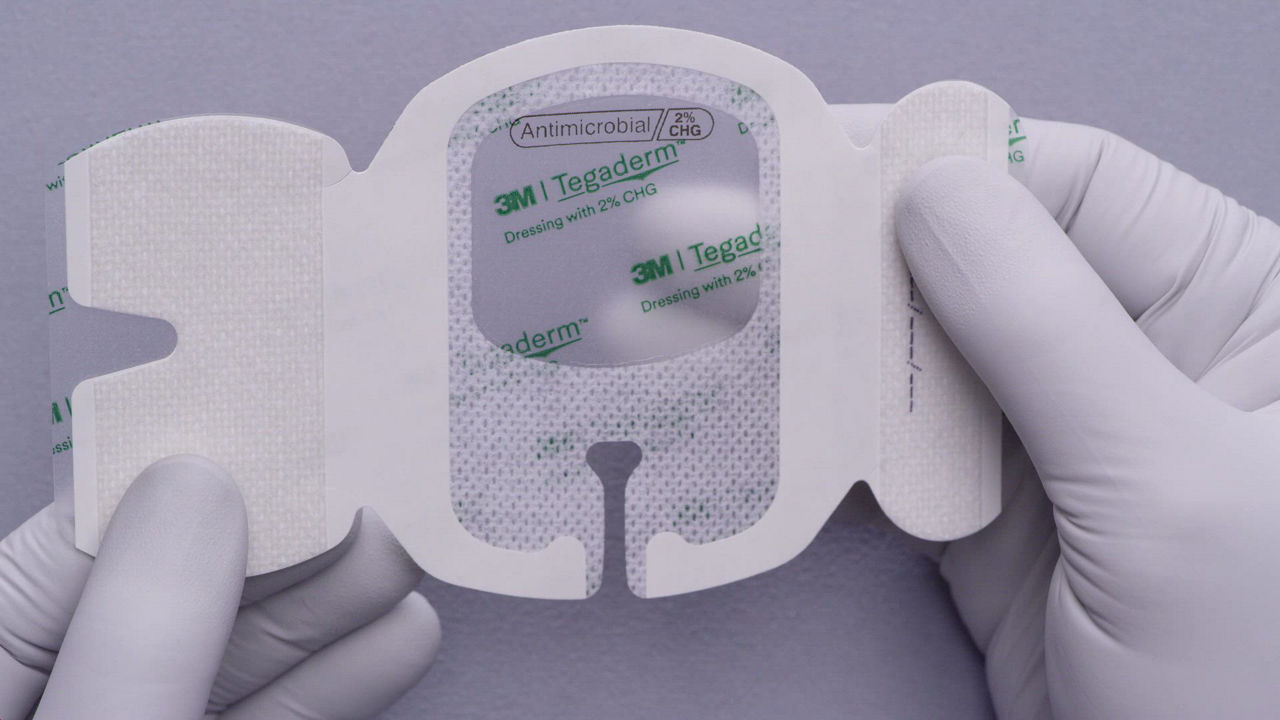
The 3M™ Tegaderm™ Antimicrobial I.V. Advanced Securement Dressing 9132 is a peripheral IV securement dressing with chlorhexidine gluconate (CHG) formulated into the dressing adhesive. Its integrated design combines antimicrobial protection with site visibility, catheter securement and consistent application for peripheral IVs.
Product details
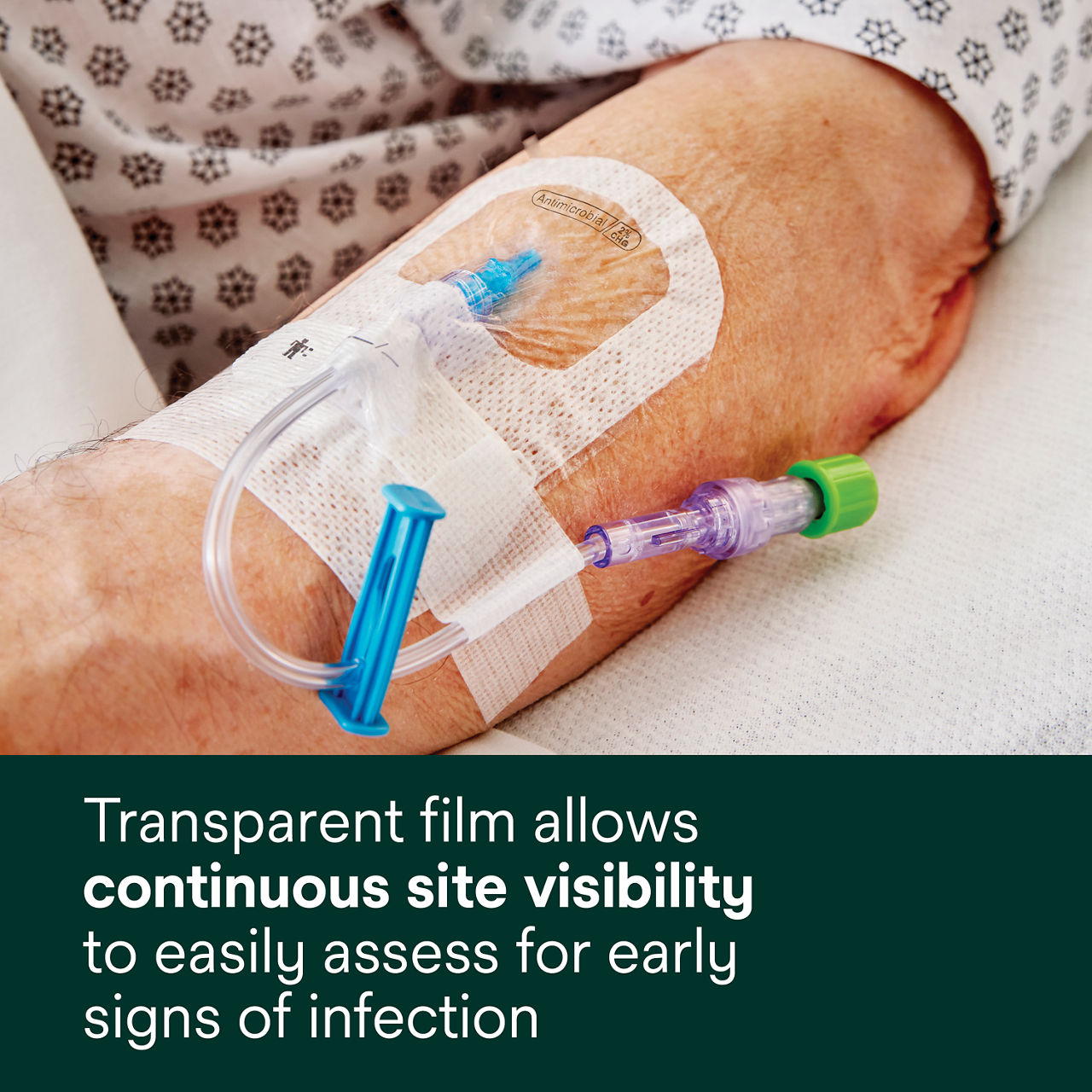
This antimicrobial IV securement dressing has a breathable, transparent film that provides a waterproof, sterile barrier to protect against external contaminants.* The CHG is formulated into the adhesive to provide antimicrobial protection without additional moisture required to activate.
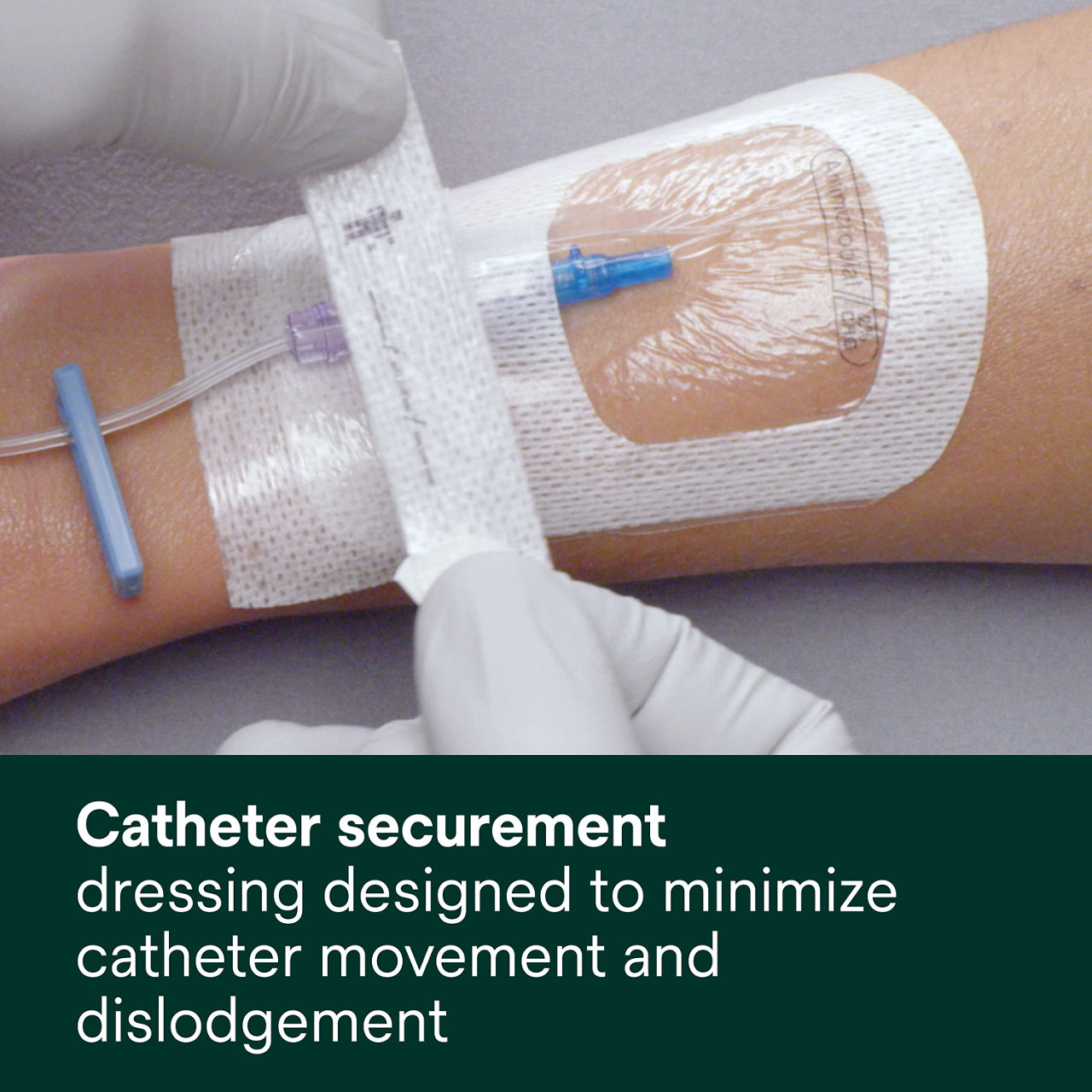
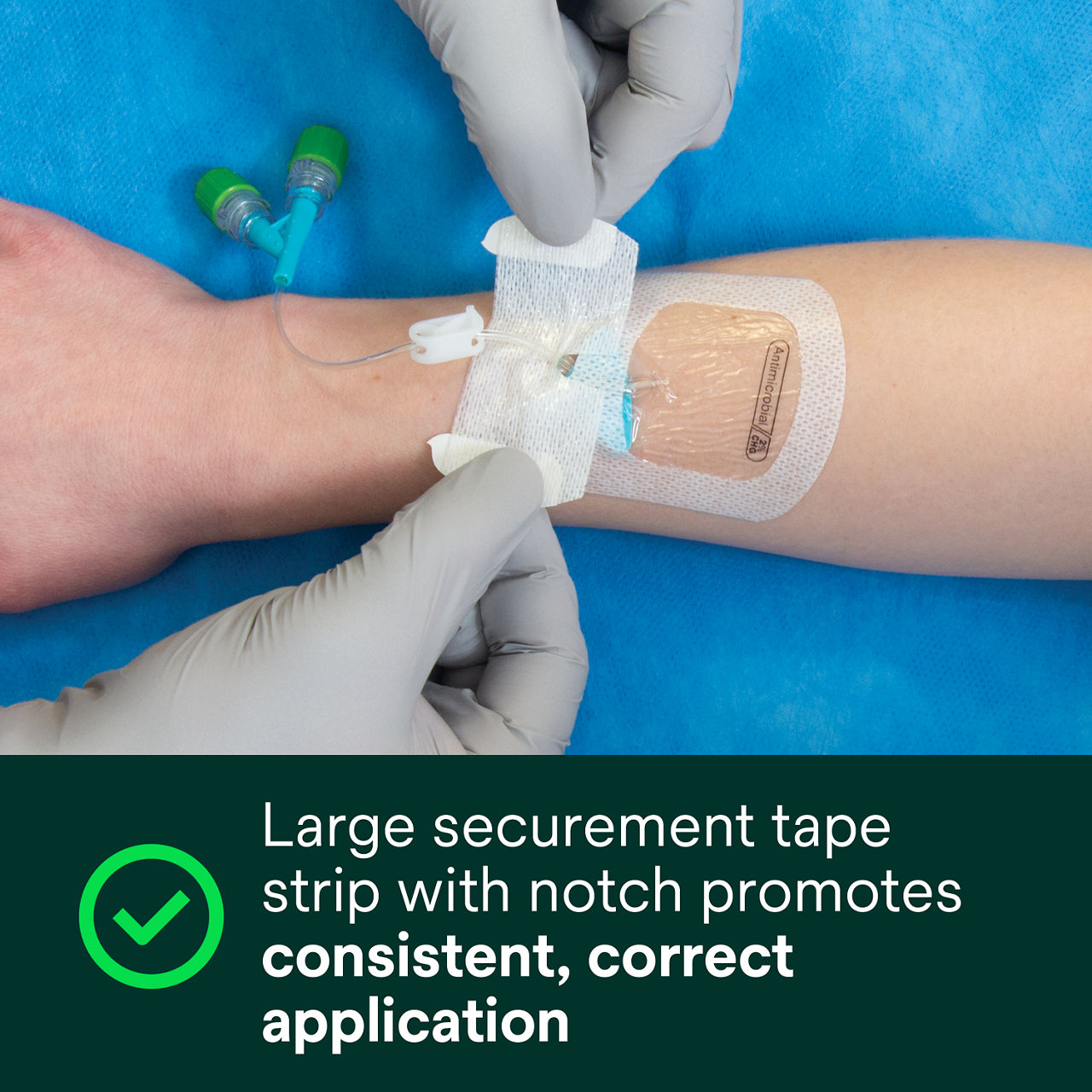
A conforming keyhole notch allows catheter lumens to fit better and stay in place, and the conforming edge border uses technology designed to reduce edge-lift. A securement tape strip with a notch promotes consistent application and enhances stabilization, and a documentation tape strip pre-printed for documenting dressing changes provides additional securement.
Note/Important Safety Warning: Not for use on premature infants or persons with known sensitivity to CHG. This device is not intended to treat, prevent or reduce catheter-related bloodstream infections (CRBSIs) or other percutaneous device-related infections. This device has not been studied in a randomized clinical study to determine its effectiveness in preventing such infections. Reference the Instructions for Use for more information.
Go go the 3M Instructions for Use Finder to view the product IFU.
- Antimicrobial IV securement dressing with 2% CHG formulated into the adhesive offers antimicrobial protection without requiring additional moisture to activate
- Integrated design combines antimicrobial protection with site visibility, catheter securement and consistent application
- Proven to suppress regrowth of skin flora for up to 7 days for sustained antimicrobial protection
- Transparent film allows continuous site visibility to easily assess for early signs of infection
- Designed to minimize catheter movement and dislodgement
- Large securement tape strip with notch promotes consistent, correct application
- Waterproof, sterile barrier protects against external contaminants* *In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage
- Looking for the non-CHG version of this IV dressing? View the 3M™ Tegaderm™ I.V. Advanced Securement Dressing, available in a wide range of sizes
Product specifications
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
3.35 in |
| Overall Width (Metric) |
Overall Width (Metric)
7 cm |
| Overall Length (Metric) |
Overall Length (Metric)
8.5 cm |
| Category Name |
Category Name
Antimicrobial IV Dressings |
| Dressing Type |
Dressing Type
Film |
| Overall Width (Imperial) |
Overall Width (Imperial)
2.76 in |