3M™ Tegaderm™ Antimicrobial Transparent Dressing 9124
About the product
The 3M™ Tegaderm™ Antimicrobial Transparent Dressing 9124 is a peripheral IV dressing with chlorhexidine gluconate (CHG) formulated into the dressing adhesive. Its integrated design combines antimicrobial protection with site visibility and breathability for peripheral IVs.
Product Details
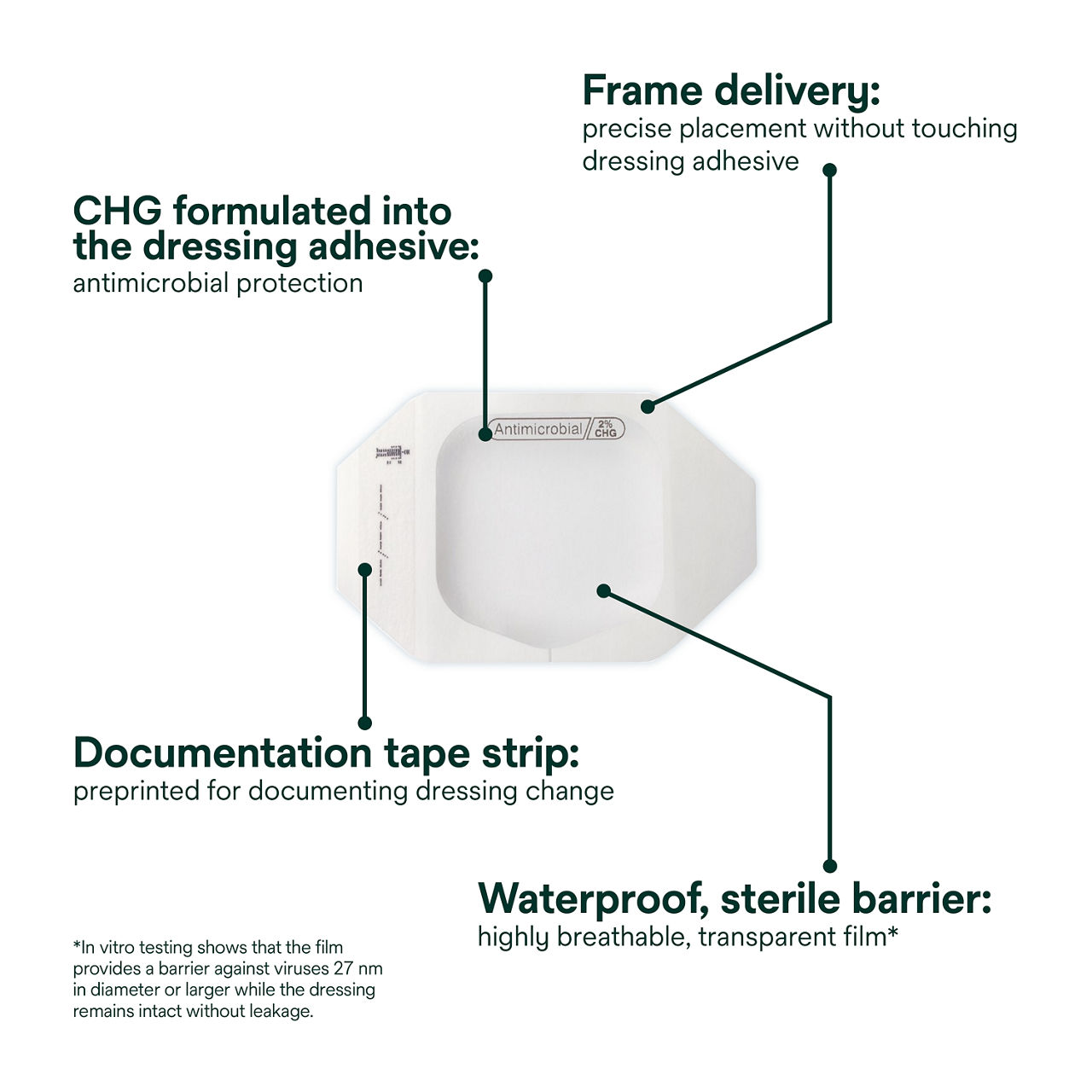
This antimicrobial IV dressing has a highly breathable, transparent film that provides a waterproof, sterile barrier to protect against external contaminants*. The CHG is formulated into the adhesive to provide antimicrobial protection without additional moisture required to activate. Frame delivery allows for precise dressing placement without touching the dressing adhesive. A pre-printed documentation tape strip for documenting dressing changes provides additional securement.
Note/Important Safety Warning: Not for use on premature infants or persons with known sensitivity to CHG. This device is not intended to treat, prevent or reduce catheter-related bloodstream infections (CRBSIs) or other percutaneous device-related infections. This device has not been studied in a randomized clinical study to determine its effectiveness in preventing such infections. Reference the Instructions for Use for more information.
Go go the 3M Instructions for Use Finder to view the product IFU.
- Antimicrobial transparent IV dressing with CHG formulated into the adhesive offers antimicrobial protection without requiring additional moisture to activate
- Its integrated design combines antimicrobial protection with site visibility and application ease for peripheral IVs
- Proven to suppress regrowth of skin flora for up to 7 days for sustained antimicrobial protection
- Transparent film allows continuous site visibility to easily assess for early signs of infection
- Integrated CHG adhesive and easy-to-learn dressing application ensures consistent IV site protection
- Waterproof, sterile barrier protects against external contaminants* *In vitro testing shows that the film provides a barrier against viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
- Interested in a securement dressing version of this product? View the 3M™ Tegaderm™ Antimicrobial I.V. Advanced Securement Dressing 9132
Product Specifications
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
2.7600 |
| Overall Width (Metric) |
Overall Width (Metric)
6.0000 |
| Overall Length (Metric) |
Overall Length (Metric)
7.0000 |
| Dressing Type |
Dressing Type
Antimicrobial Transparent |
| Overall Width (Imperial) |
Overall Width (Imperial)
2.3600 |