3M™ Tegaderm™ CHG Chlorhexidine Gluconate Gel Pad
About the product
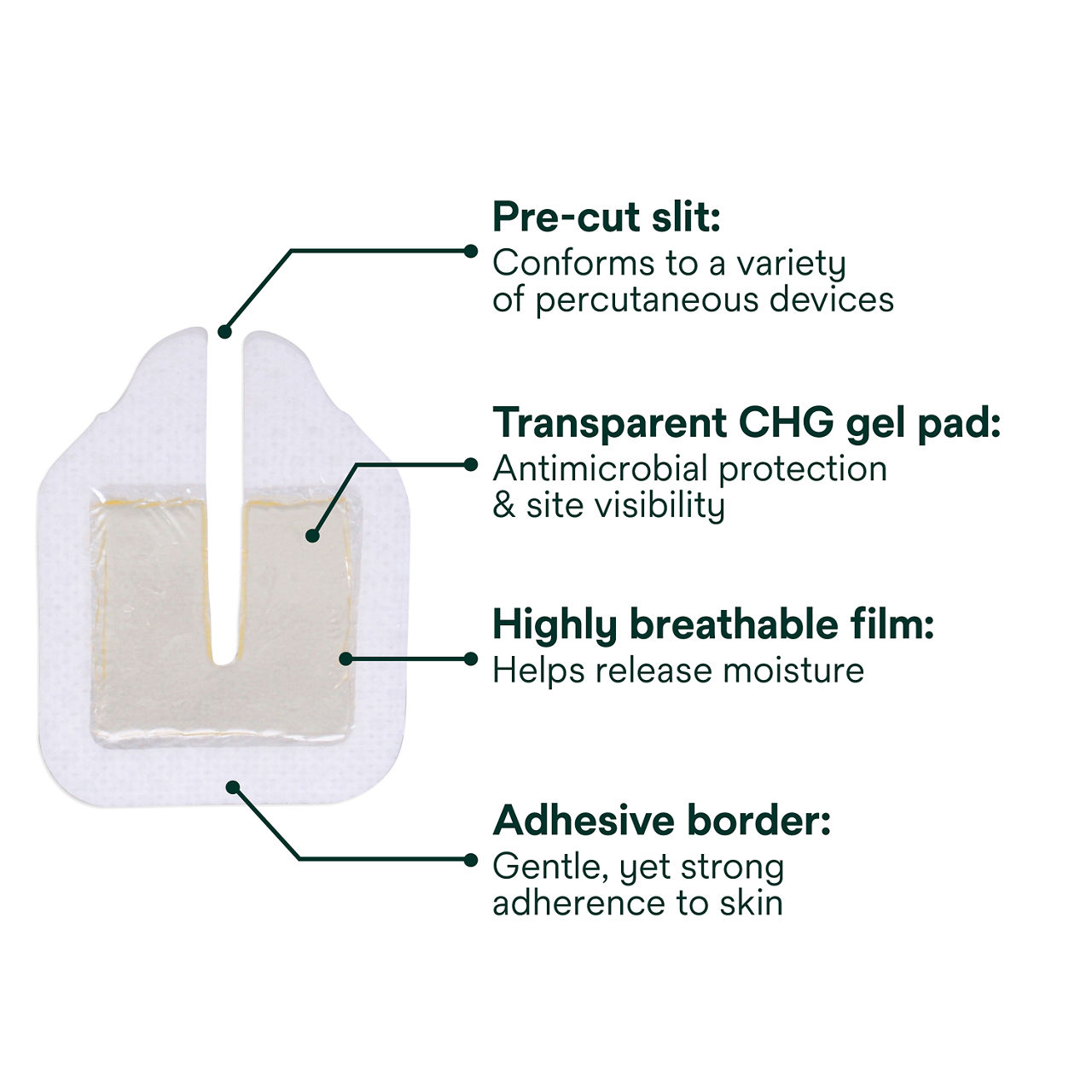
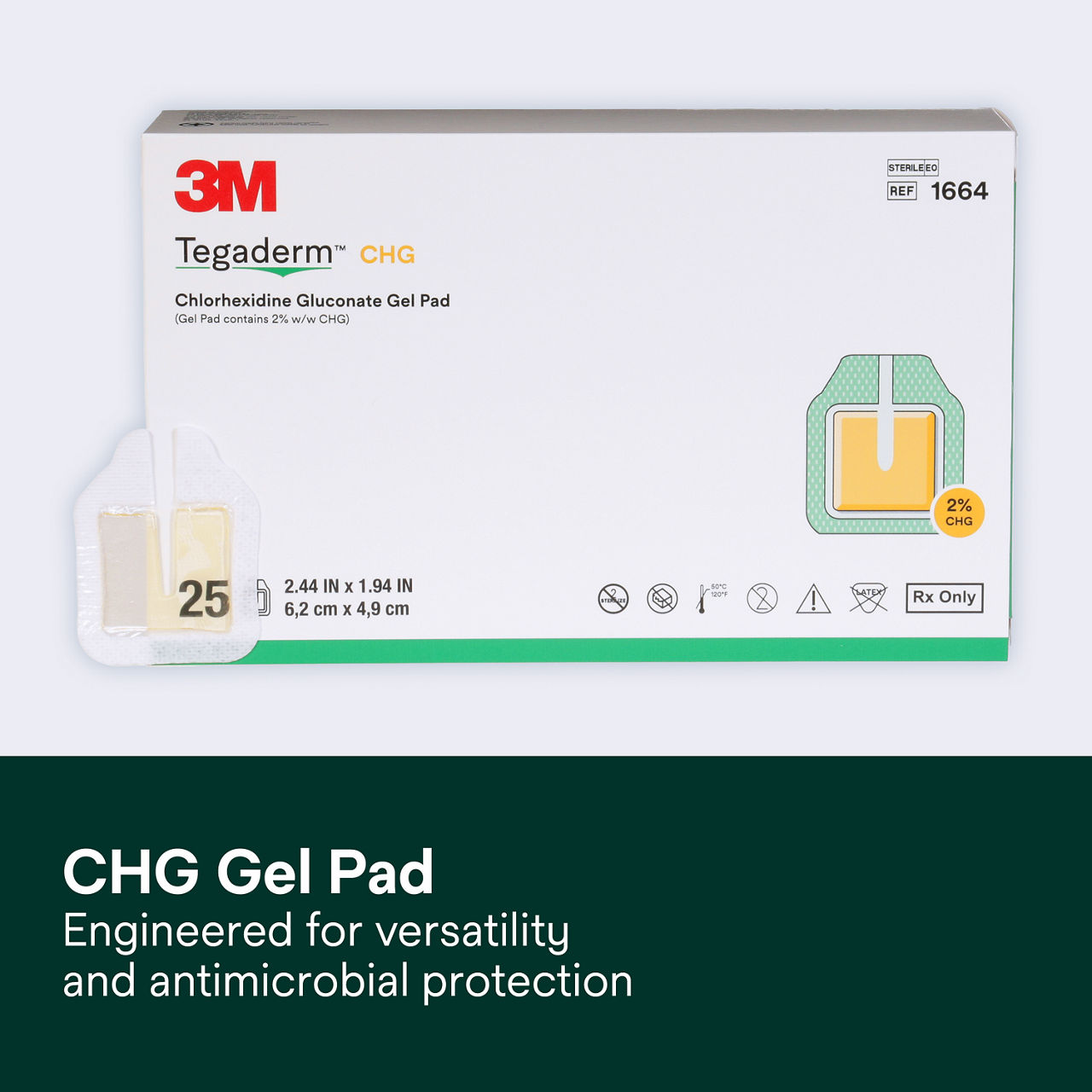
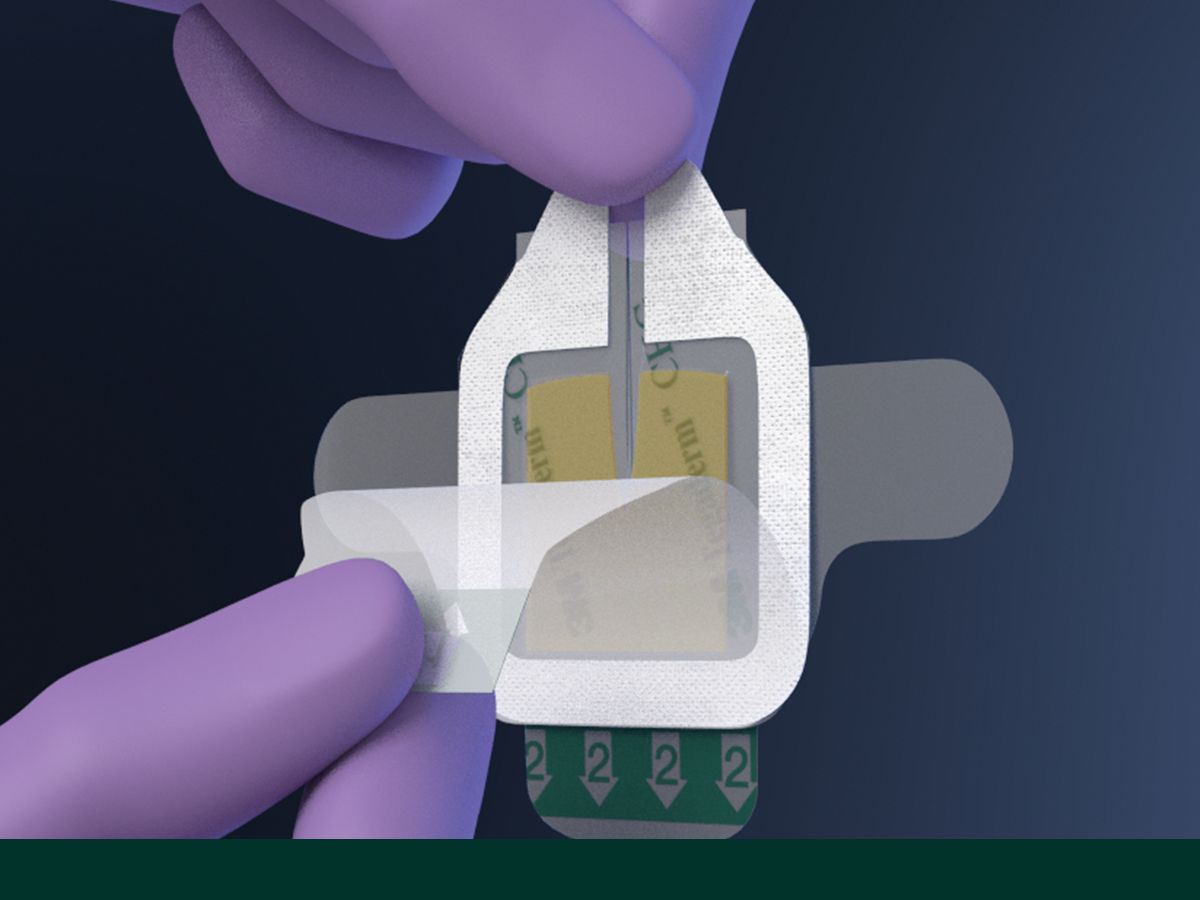
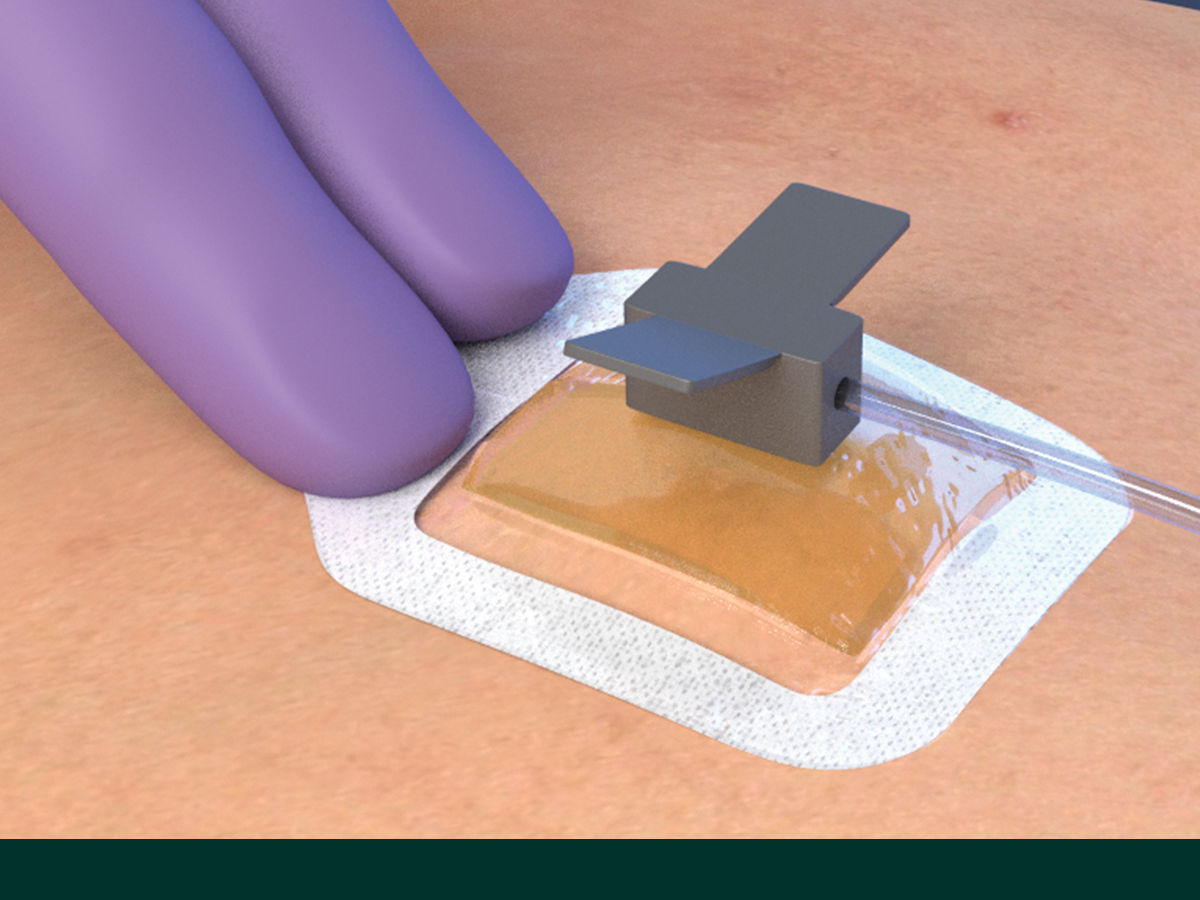
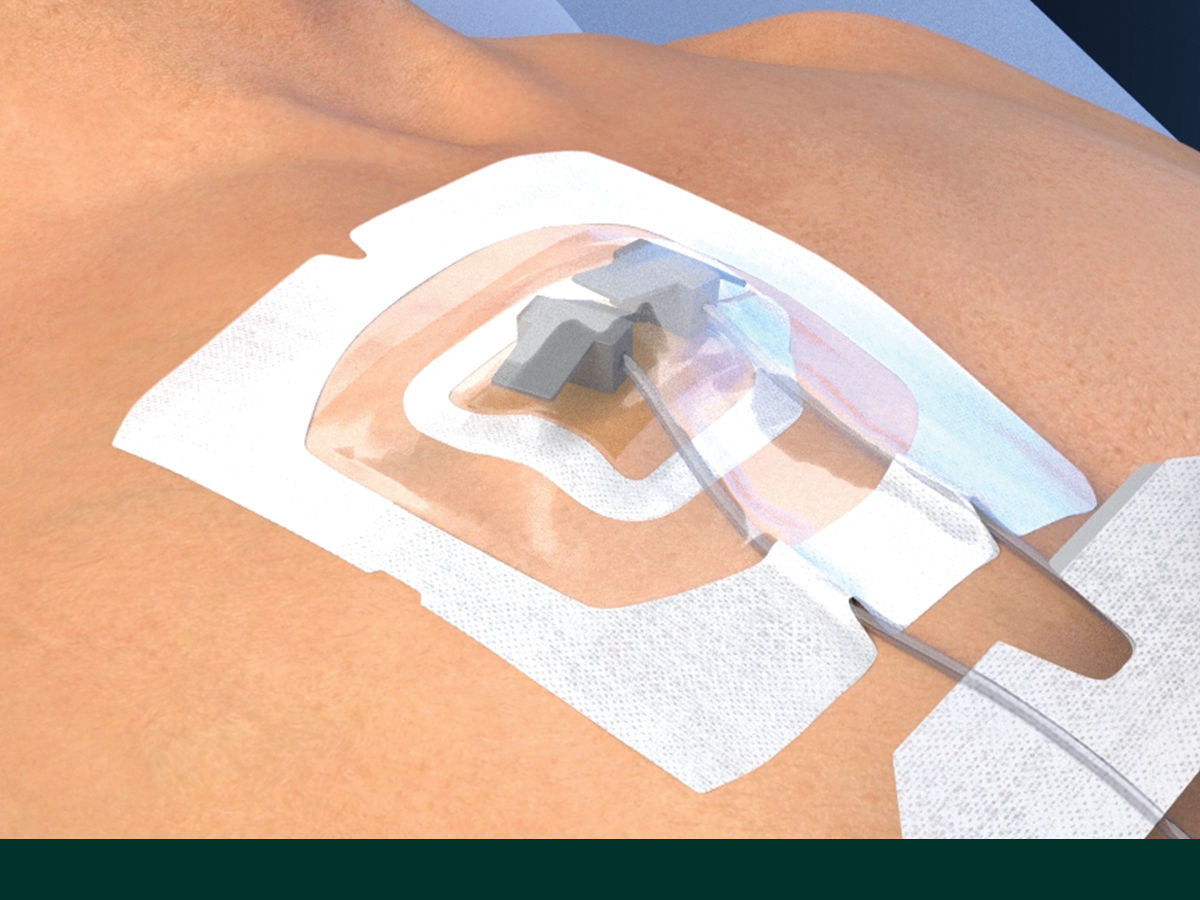
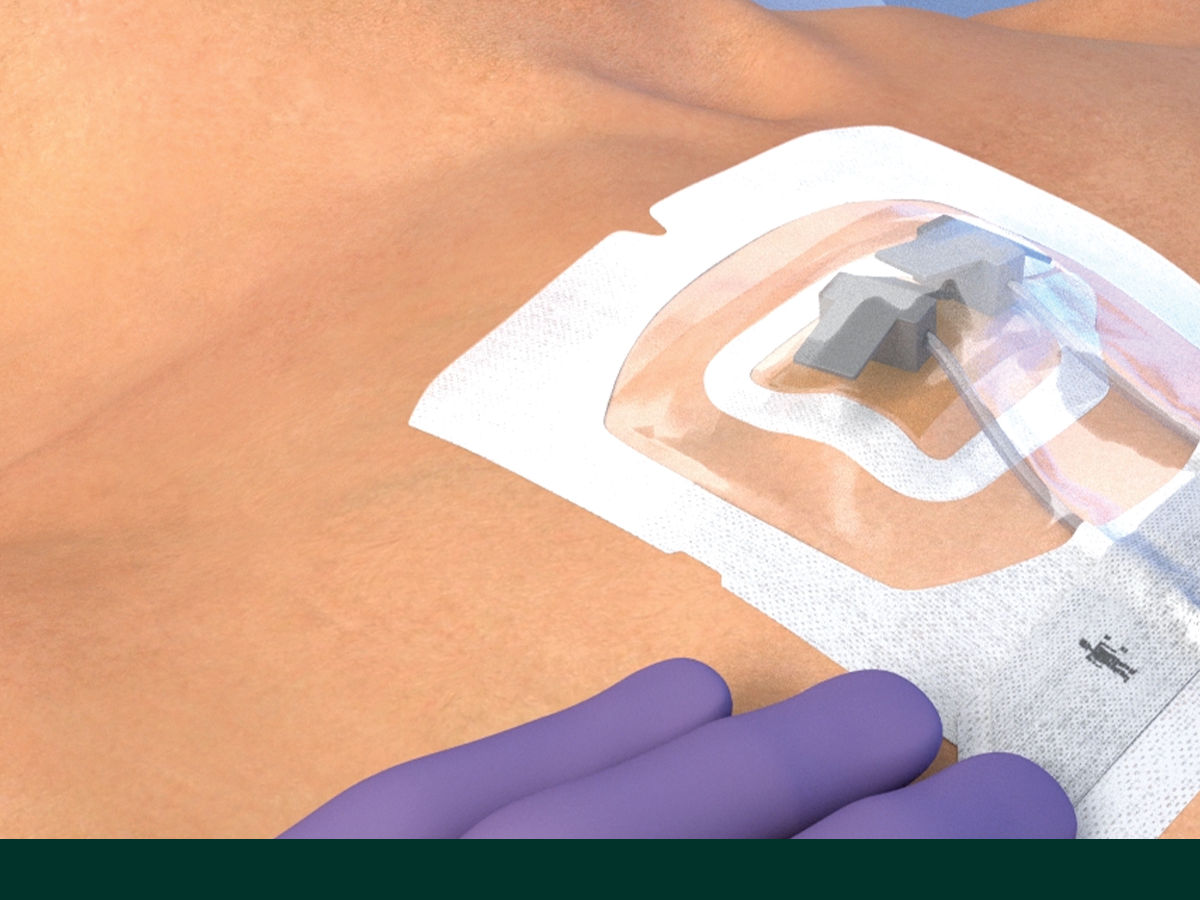
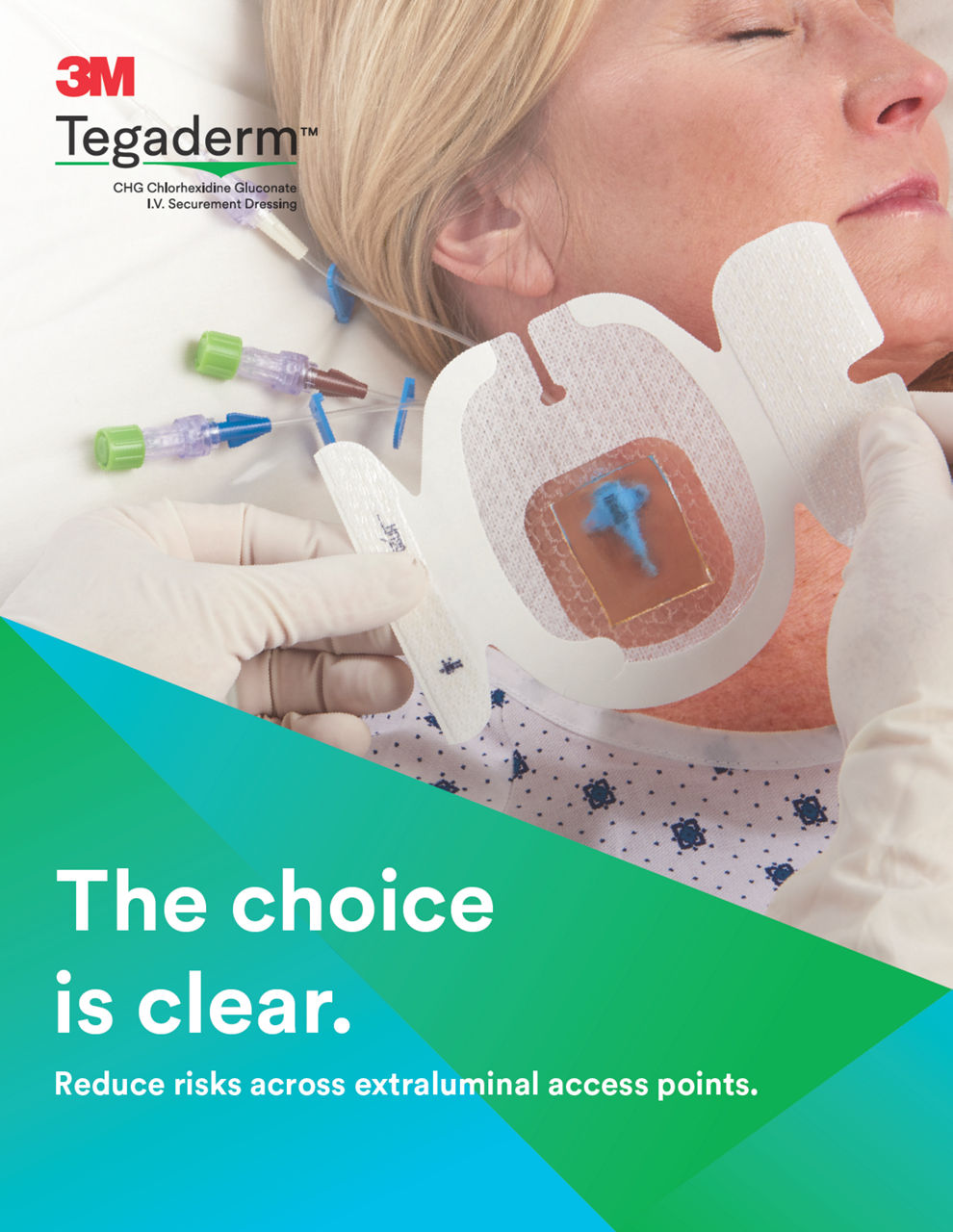
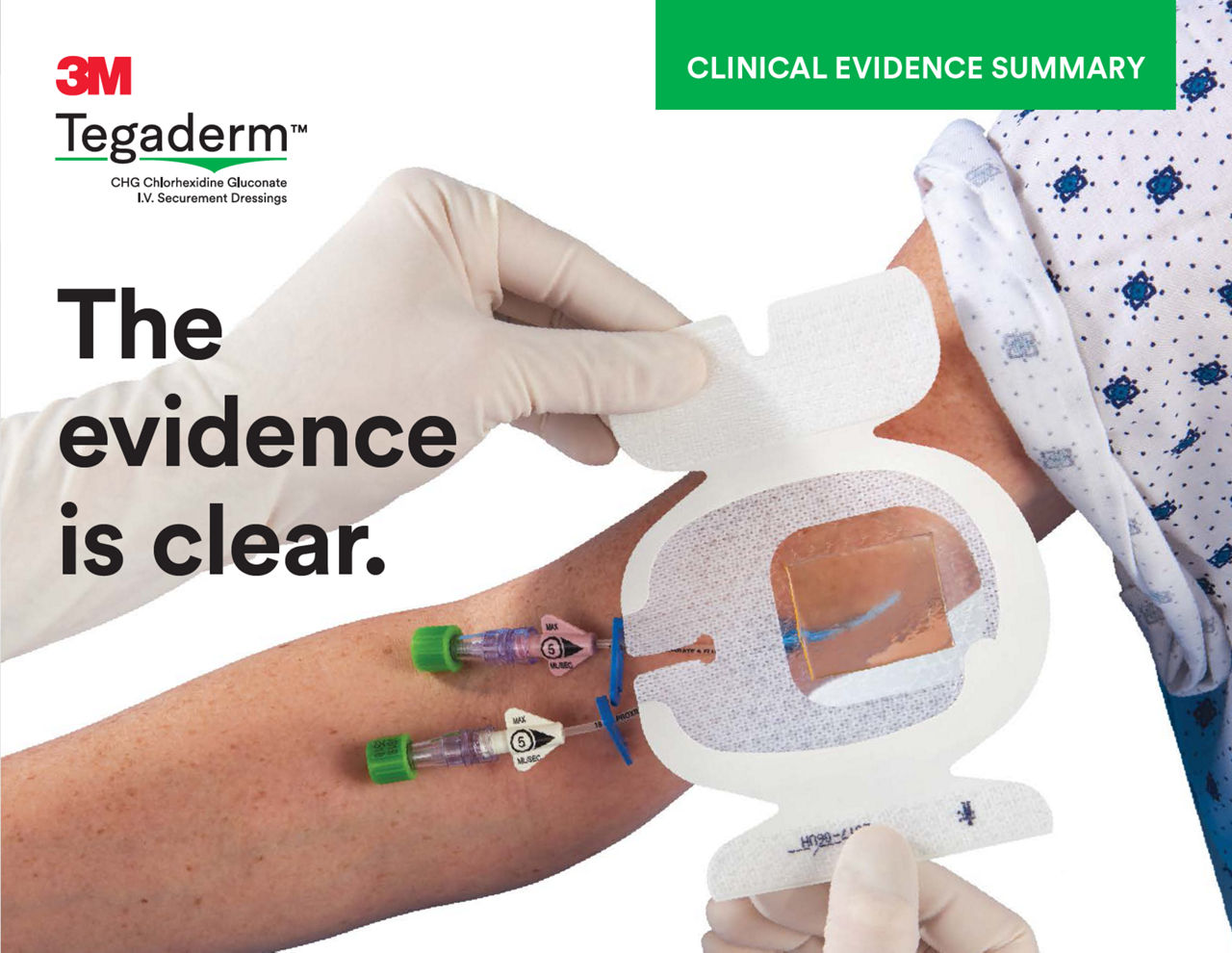
The Tegaderm CHG Chlorhexidine Gluconate Gel Pad 1664 is an antimicrobial (CHG) gel pad designed to protect insertion sites and conform around a wide variety of percutaneous devices. This transparent CHG gel pad combines versatility with site visibility and antimicrobial protection.
Product details
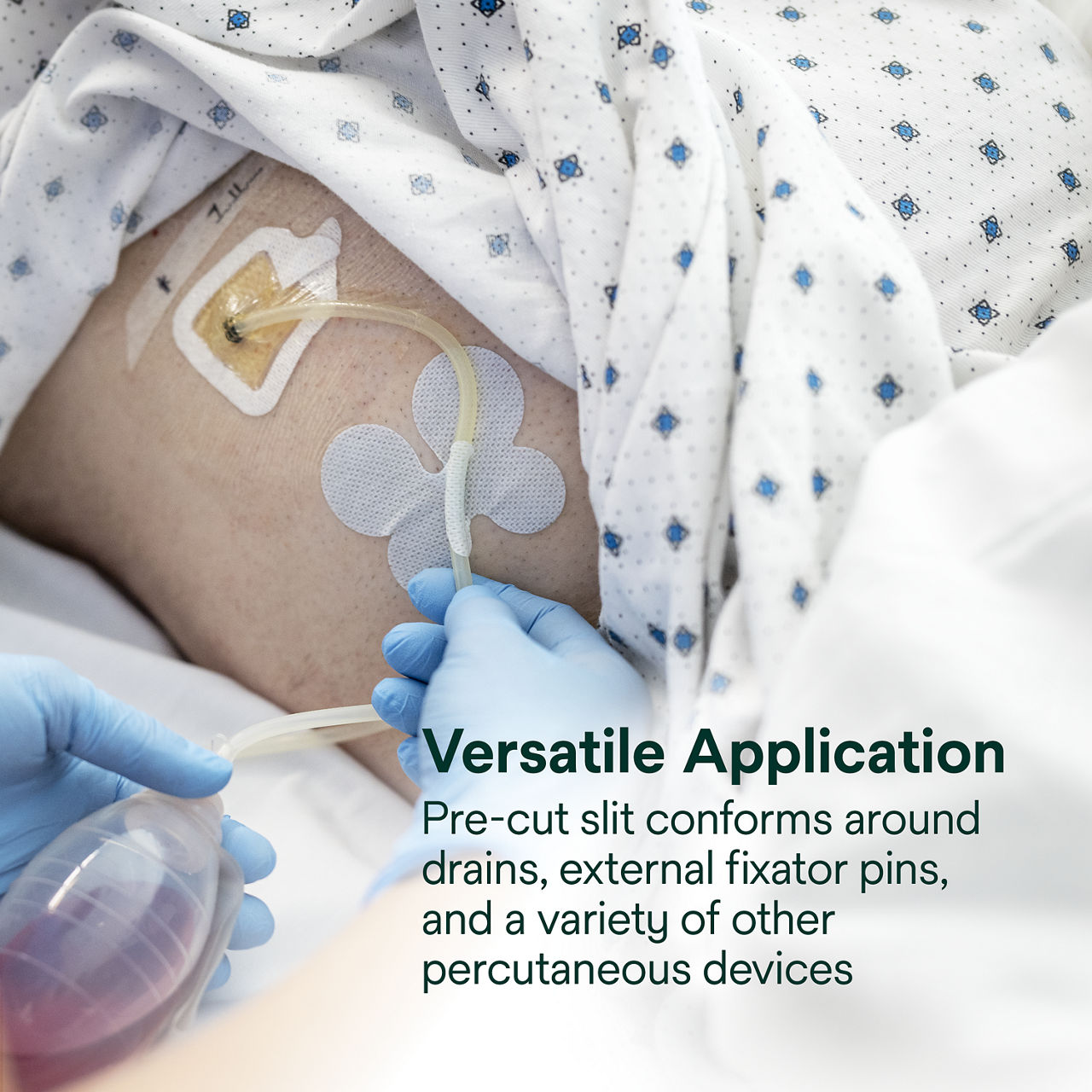
To see application for drains, external fixator pins, and other percutaneous devices, .
Note/Important Safety Warning: Do not use Tegaderm™ CHG Gel Pad on premature infants or infants younger than two months of age. Use of this product on premature infants may result in hypersensitivity reactions or necrosis of the skin. The safety and effectiveness of Tegaderm™ CHG Gel Pad has not been established in children under 18 years of age. For full prescribing information, see the
Instructions for Use. RX Only.Go to the 3M Instructions for Use Finder to view the product IFU.
- Standalone CHG gel pad engineered for versatility and antimicrobial protection
- Tegaderm CHG is intended to reduce catheter-related bloodstream infections (CRBSIs) in patients with central venous or arterial catheters
- CHG gel pad provides immediate and continuous antimicrobial activity – reducing skin flora and preventing its re-growth – for up to 7 days
- Pre-cut slit conforms around drains, external fixator pins, implanted venous ports and a wide variety of other percutaneous devices (up to 40 FR)
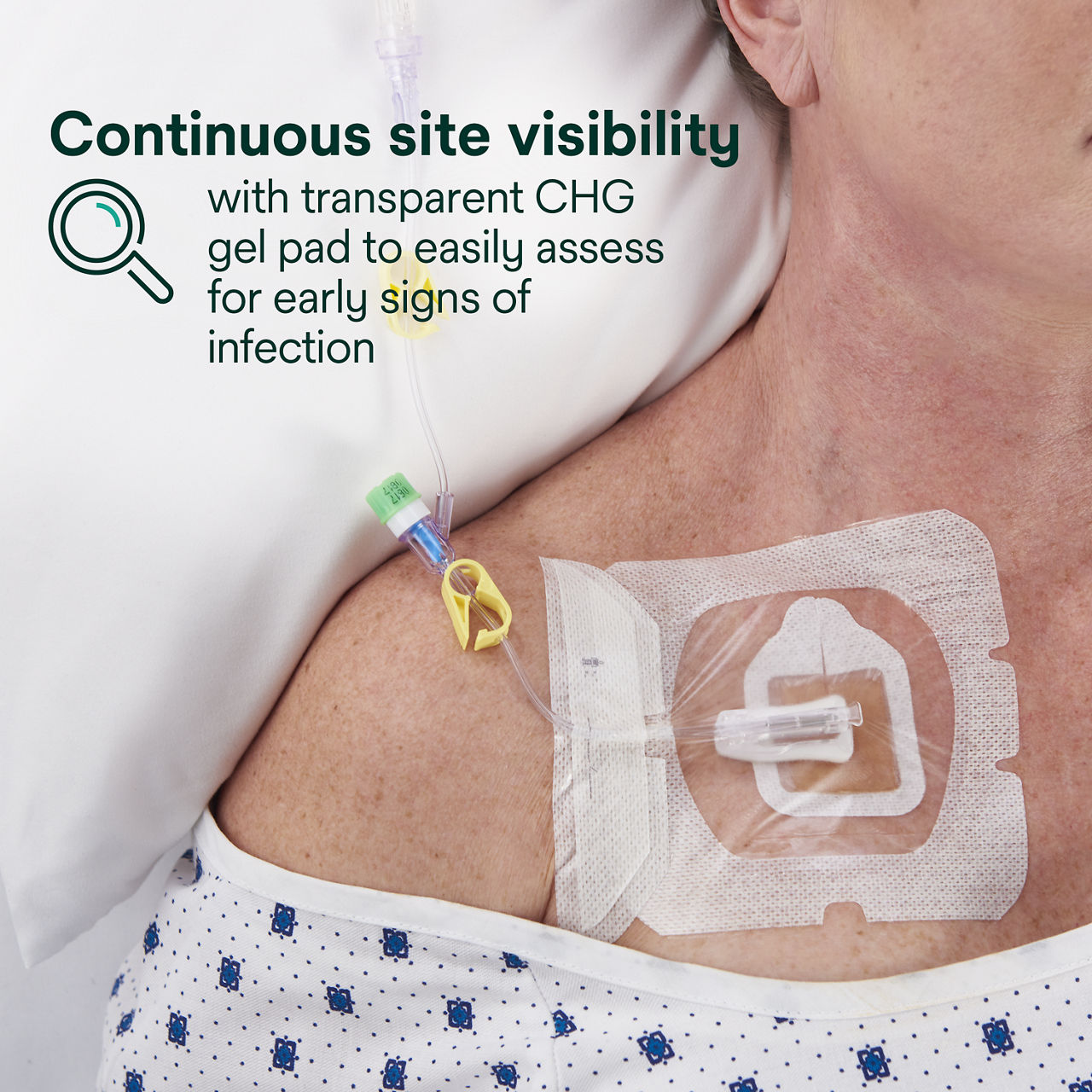
- Transparent CHG gel pad allows continuous site visibility to easily assess for early signs of infection
- Adhesive border ensures gentle, yet strong adherence to skin
- Absorbs low amount of exudate* *Bench testing shows that CHG gel can absorb 8x its weight in saline and 3x its weight in blood
Legal
* Bench testing shows that CHG gel can absorb 8x its weight in saline and 3x its weight in bloodProduct specifications
| Overall Length (Imperial) |
Overall Length (Imperial)
2.44 in |
| Overall Width (Metric) |
Overall Width (Metric)
4.9 cm |
| Overall Length (Metric) |
Overall Length (Metric)
6.2 cm |
| Dressing Type |
Dressing Type
Film |
| Category name |
Category name
Antimicrobial IV Dressings |
| Trademark2 |
Trademark2
Tegaderm™ |
| Overall Width (Imperial) |
Overall Width (Imperial)
1.93 in |
| categoryName |
categoryName
Antimicrobial IV Dressings |