IV dressings
The right IV dressing matters
We offer a comprehensive range of reliable intravenous (IV) dressings, specifically designed for all catheter types. Our broad portfolio of IV dressings makes it easy for you to choose the right product for your patient. We can help you deliver compassionate care with evidence-based products to help prevent the risks of costly complications and improve patient satisfaction.

The science behind our IV dressings
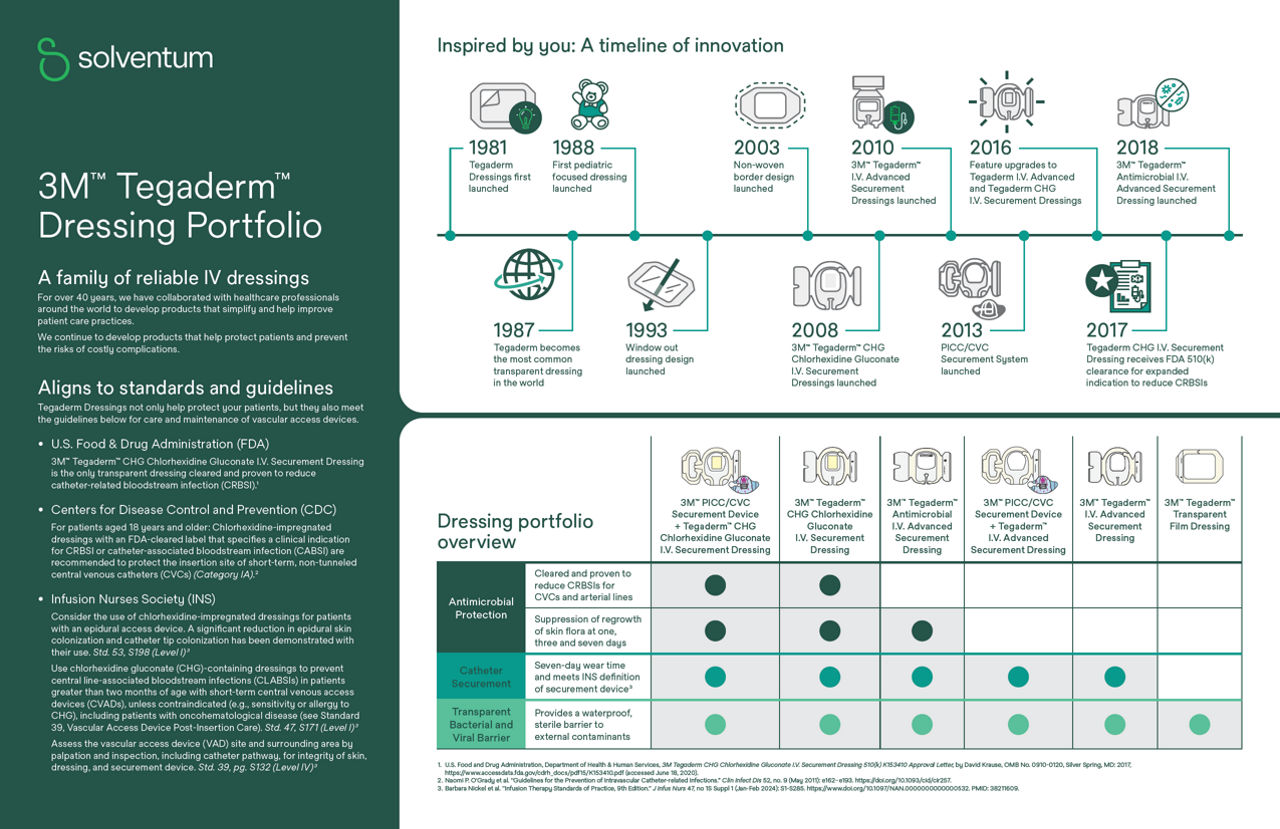
Backed by 40 years of IV care science and innovation, Tegaderm IV Dressings are designed to provide breakthrough solutions that protect, support and help your patients heal. Dressing categories include:
Antimicrobial protection
Includes IV dressings containing 2% CHG and proven to suppress regrowth of skin flora for up to 7 days for sustained antimicrobial protection1 . Our Tegaderm CHG I.V. securement dressing solution meets the Infusion Nurse Society's highest-level recommendation to use chlorhexidine-impregnated dressings to reduce the risk of catheter-associated bloodstream infection (CABSI).2
Catheter securement
Designed to provide reliable securement, site visibility and breathability all in one product. These dressings are comfortable to wear for up to seven days and meet the Infusion Nurses Society definition of a securement device.2
Bacterial and viral barrier*
The original Tegaderm Dressing was launched in 1981 and provides a waterproof, sterile barrier to external contaminants with transparency for easy monitoring.

Tegaderm CHG IV securement dressings

The choice is clear
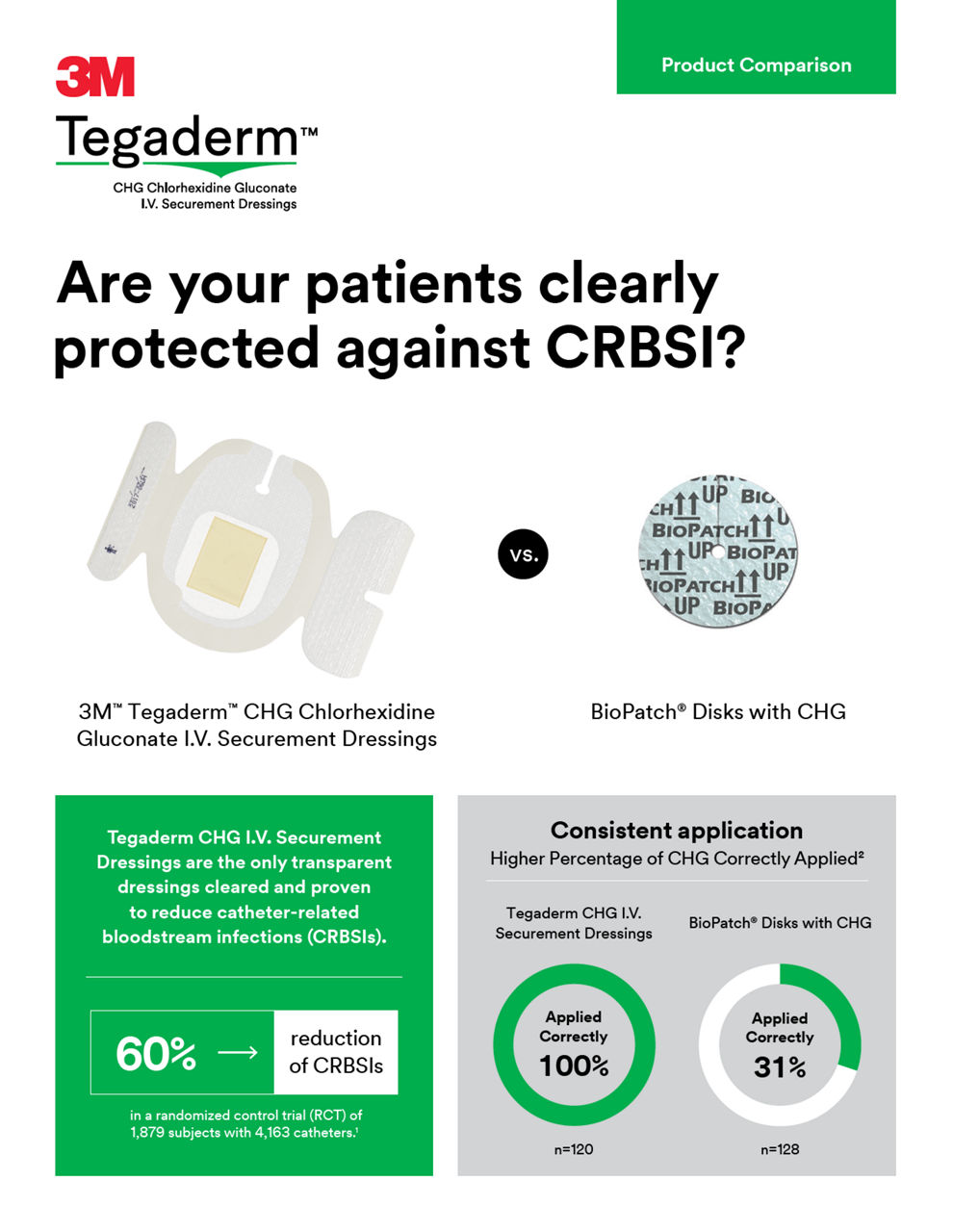
3M™ Tegaderm™ CHG Chlorhexidine Gluconate IV Securement Dressings are the only transparent dressing cleared by the Food and Drug Administration (FDA) to reduce catheter-related bloodstream infections (CRBSI) and vascular catheter colonization, that aligns with evidence-based guidelines and practice standards.
How Tegaderm CHG Chlorhexidine Gluconate IV securement dressings can benefit you and your patients:
Infection reduction — provides antimicrobial protection
Cleared and clinically proven to reduce CRBSI. Meets standards and guidelines, including the Centers for Disease Control and Prevention (CDC) Guidelines recommendation for the use of chlorhexidine-impregnated dressing with a FDA indication to reduce CRBSI.
Site visibility — easily monitor IV sites
Transparent dressing and gel pad enable early identification of potential complications at the IV site and meet Infusion Nurses Society (INS) recommendation to assess the IV site and surrounding area by visual inspection.1
Catheter securement — securely hold catheters in place
Designed to minimize catheter movement and dislodgement and meets the INS definition of an Engineered Stabilization Device (ESD).3
Ease of use — easily apply and remove
The integrated CHG gel pad and dressing are designed to ensure standardized, correct application.4
How Tegaderm CHG IV dressings align to industry guidelines
Align your infection prevention protocols with evidence-based guidelines and practice standards to help minimize IV site complications. Many of the world’s well-regarded organizations recommend the use of the Tegaderm CHG IV securement dressings to help reduce vascular catheter colonization and other complications — helping to deliver the highest level of care to your patients.
Tegaderm CHG products
Tegaderm CHG resources
Turn to our educational materials to help you meet a variety of clinical goals. Learn about solutions based on your planned application.
Tegaderm IV advanced securement dressings
Reliable securement solutions that help prevent complications
Catheter movement is a prevalent problem. Up to one-third of vascular access devices become dislodged.7 Properly securing IV lines is a key part of maintaining quality care and patient satisfaction. Help stabilize catheters and minimize movement and dislodgement with 3M™ Tegaderm™ IV Advanced Securement Dressing and PICC CVC Securement Device + Tegaderm IV Advanced Securement Dressing.
Tegaderm IV Advanced Securement Dressings can help facilities manage IV site care costs by potentially reducing the number of dressing changes and catheter restarts. The transparent film allows for continuous monitoring of the insertion site. The dressing may be worn for the life of a PIV catheter and provides up to 7 days of wear time for CVCs.8 A waterproof film coating on all pieces resists soiling and provides a barrier to external contaminants, including liquids, bacteria and viruses.*
The PICC/CVC securement device + Tegaderm IV advanced securement dressing is a sutureless securement system that provides reliable PICC/CVC securement without sacrificing patient comfort. It includes an adhesive catheter securement device plus an IV securement dressing to help you avoid the clinical, financial and emotional costs of PICC/CVC complications, including needlestick injuries.
* In vitro testing shows that the transparent film of Tegaderm brand dressings provide a viral barrier for viruses 27 nm in diameter or larger while the dressing remains intact without leakage.
Tegaderm transparent film dressings
A versatile, transparent barrier to contaminants
For more than 40 years, our 3M™ Tegaderm™ transparent film dressings have been recognized as the best practice recommendation for the minimum standard of IV care and maintenance.10 They provide a versatile, waterproof sterile barrier to external contaminants including bacteria, viruses*, blood and bodily fluids. These transparent dressings provide full site visibility for precise placement and continuous monitoring to help minimize unnecessary dressing changes.
The 3M™ Tegaderm™ Diamond Pattern Film Dressing and 3M™ Tegaderm™ HP (Holding Power) Transparent Film Dressing are ideal for moist conditions because they have specialized features, providing better wear for humid conditions or with diaphoretic patients.9
* In vitro testing shows that the transparent film of Tegaderm brand dressings provide a viral barrier for viruses 27 nm in diameter or larger while the dressing remains intact without leakage. 334–343.
Resources
IV dressing training videos
- Bashir MH, Olson LK, Walters SA. Suppression of regrowth of normal skin flora under chlorhexidine gluconate dressings applied to chlorhexidine gluconate-prepped skin. Am J Infec Control. 2012;40:344-348.
- Nickel B, Gorski L, Kleidon T, Kyes A, DeVries M, Keogh S, Meyer B, Sarver MJ, Crickman R, Ong J, Clare S, Hagle ME. Infusion Therapy Standards of Practice, 9th Edition. J Infus Nurs. 2024 Jan-Feb 01;47(1S Suppl 1):S1-S285. doi: 10.1097/NAN.0000000000000532. PMID: 38211609. © 2024 Infusion Nurses Society (INS). Excerpts used by explicit permission from INS. No endorsement is implied or given.
- Infusion Nurses Society (INS): Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion Therapy Standards of Practice. J Infus Nurs. 2016;39(suppl 1):S1-S59.
- Kohan CA, Boyce JM. A Different Experience with Two Different Chlorhexidine Gluconate Dressings for Use on Central Venous Devices. Am J Infect Control. 2013;41(6):S142–S143
- U.S. Food and Drug Administration, Department of Health & Human Services. 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Securement Dressing 510(k) K153410 approval letter, May 15, 2017. Retrieved June 18, 2020 from https://www.accessdata.fda.gov/cdrh_docs/pdf15/K153410.pdf
- Centers for Disease Control and Prevention (CDC): O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193
- Jackson A. Retrospective comparative audit of two peripheral IV securement dressings. Br J Nurs. 2012;21(2 Suppl):1015.
- O’Grady, N et al. (2011). Guidelines for the Prevention of Intravascular Catheter-Related Infections, 2011.
- 3M Data on file (05-012089).
- Gorski LA, Hadaway L., Hagle ME, et al. Infusion Therapy Standards of Practice. J Infus Nurs. 2021;42, Standard 42.4, Practice Recommendation K1.