3M Skin and Nasal Antiseptic
Enhance patient safety in the ICU and the OR

Make Skin and Nasal Antiseptic part of your nasal decolonization protocol
Confidence in surgery matters. Take control of your patients’ nasal decolonization in both the intensive care unit (ICU) and operating room (OR) with Skin and Nasal Antiseptic.
Designed to help address the rising concern of SSIs that can be caused by bacteria in the nares, this solution is a key element of patient preoperative skin preparation. By reducing the risk factors that contribute to SSIs, you can make a significant impact on patient outcomes. Using Skin and Nasal Antiseptic before surgery and in the ICU helps reduce bacterial load, including Staphylococcus aureus, which is the leading cause of SSIs among adult and pediatric patients. By aligning with CDC, SHEA, IDSA, APIC and other leading organizations, you can help prevent SSIs.
We share your passion for infection prevention. Let us help you protect every patient, every time.
Proven efficacy. Simple application
Make a real difference in your presurgical infection prevention protocol with Skin and Nasal Antiseptic (Povidone-Iodine Solution 5% w/w [0.5% available iodine] USP) Patient Preoperative Skin Preparation. Designed to prepare the skin prior to surgery and help reduce bacteria that potentially can cause skin infection.
- Simple, one-time application
- Helps reduce bacteria that potentially can cause skin infections
- 99.9% decrease in bacteria within 10 minutes and sustained reduction for up to 6 hours
- Kills mupirocin non-susceptible MRSA and vancomycin-intermediate MRSA1 *
Strength of our antiseptics
*When evaluated using an in vitro test method. The clinical significance of in vitro data is unknown.
Why choose Skin and Nasal Antiseptic?
10+ years since initial launch
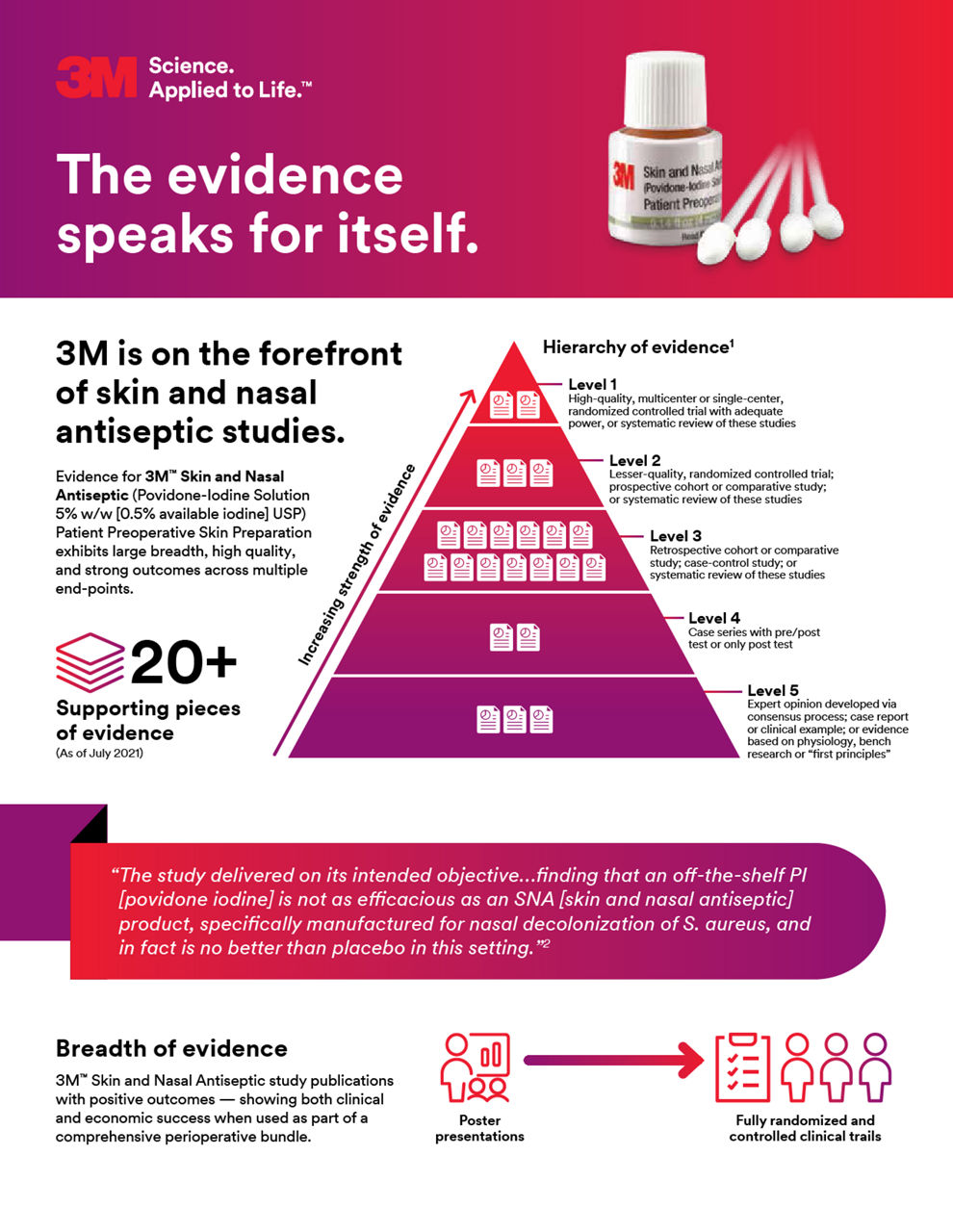
Our Skin and Nasal Antiseptic is supported by evidence that met or exceeded the hypotheses across multiple endpoints including microbiological impacts that were associated with infection risk reduction outcomes and economic success when used as part of a comprehensive perioperative solution.
Choose our evidence-based antiseptic nasal swabs as part of your nasal decolonization protocol to prepare the skin prior to surgery and help reduce bacteria that potentially can cause skin infections.
*(Ex vivo porcine model): Anderson M, David M et al. 2015. Efficacy of Skin and Nasal Povidone-Iodine Preparation against mupirocin resistant MRSA and Staphylococcus aureus with the anterior nares, Antimicrob Agents Chemother. pii: AAC.04624-14.
Resources
References:
- Karau M, Ballard A et al. “3M Skin and Nasal Antiseptic and 3M DuraPrep Surgical Solution Bactericidal Activity Against Methicillin-Resistant Staphylococcus aureus.” American Society for Microbiology General Meeting, Boston MS, May 2014.
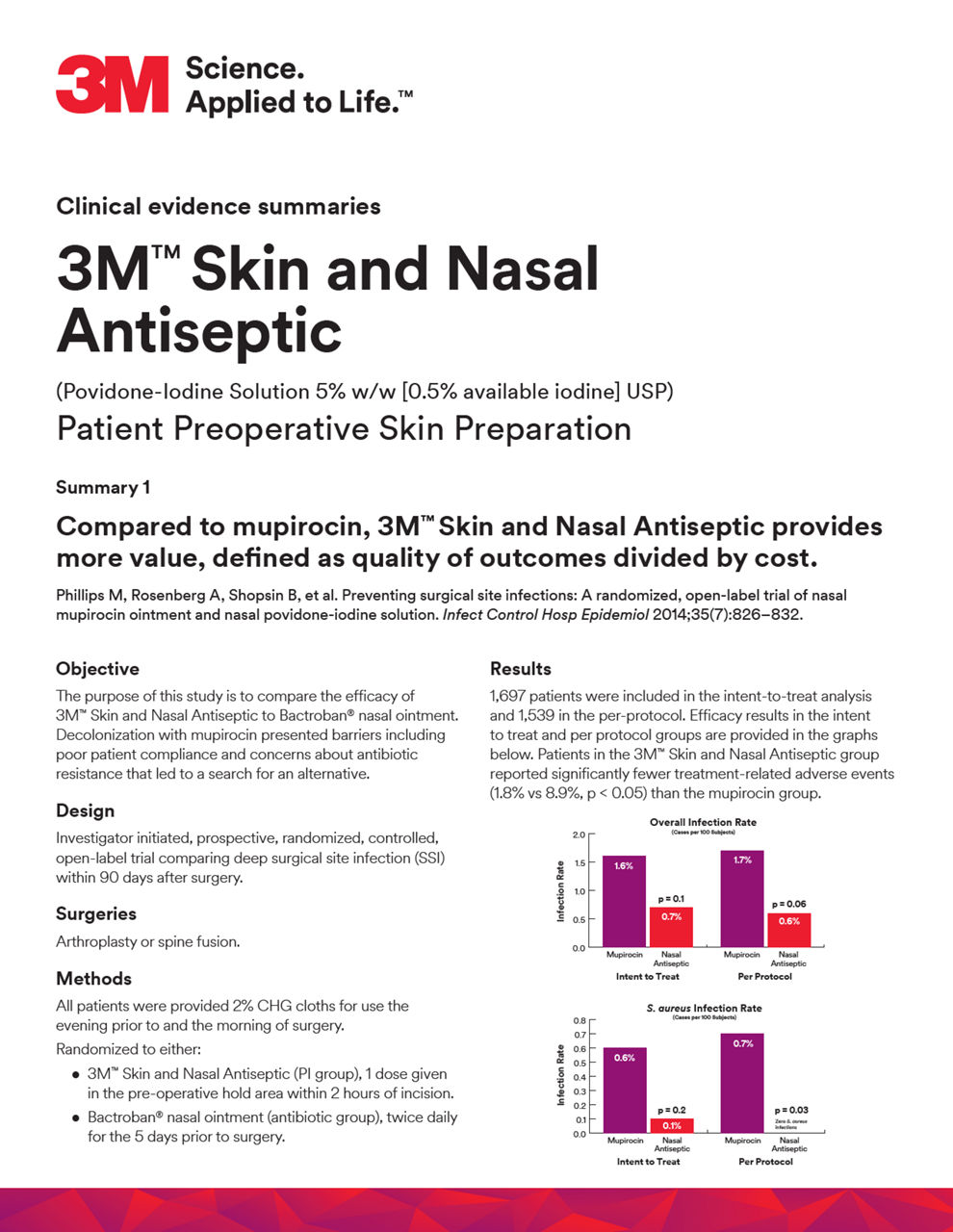
- Phillips M, Rosenberg A, Shopsin B, et al. Preventing surgical site infections: A randomized, open-label trial of nasal mup. ointment and nasal povidone-iodine solution. Infect Control Hosp Epidemiol. 2014;35:826-32.
- 3M data on file: EM-05-011017.
- 3M data on file.
- 3M data on file: EM-05-159073.