PREVENA PLUS™ 125 Therapy Unit, PRE4000US, 3 Kits/Cs
About the product
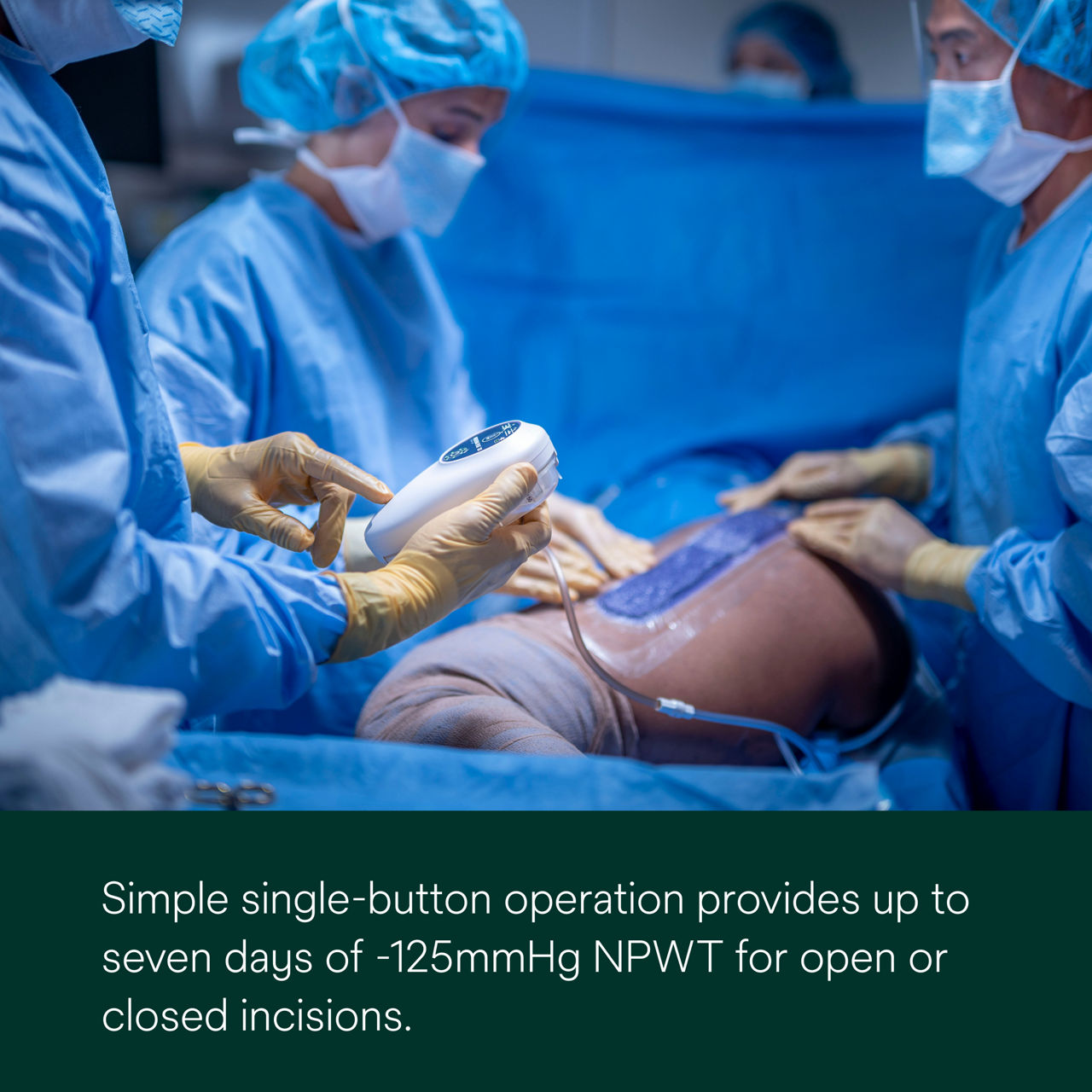
Simplify postoperative wound management with the 3M™ Prevena™ Plus Therapy Unit (7-Day). Designed for closed incisions and small-to-medium open wounds, this single-use, portable device delivers up to seven days of continuous -125mmHg negative pressure wound therapy with the push of a button. This therapy unit can be paired with a range of select 3M NPWT dressings.
Product details
As a healthcare provider, your goal is to support and care for your patients through their recovery, ensuring it goes as smoothly and efficiently as possible.
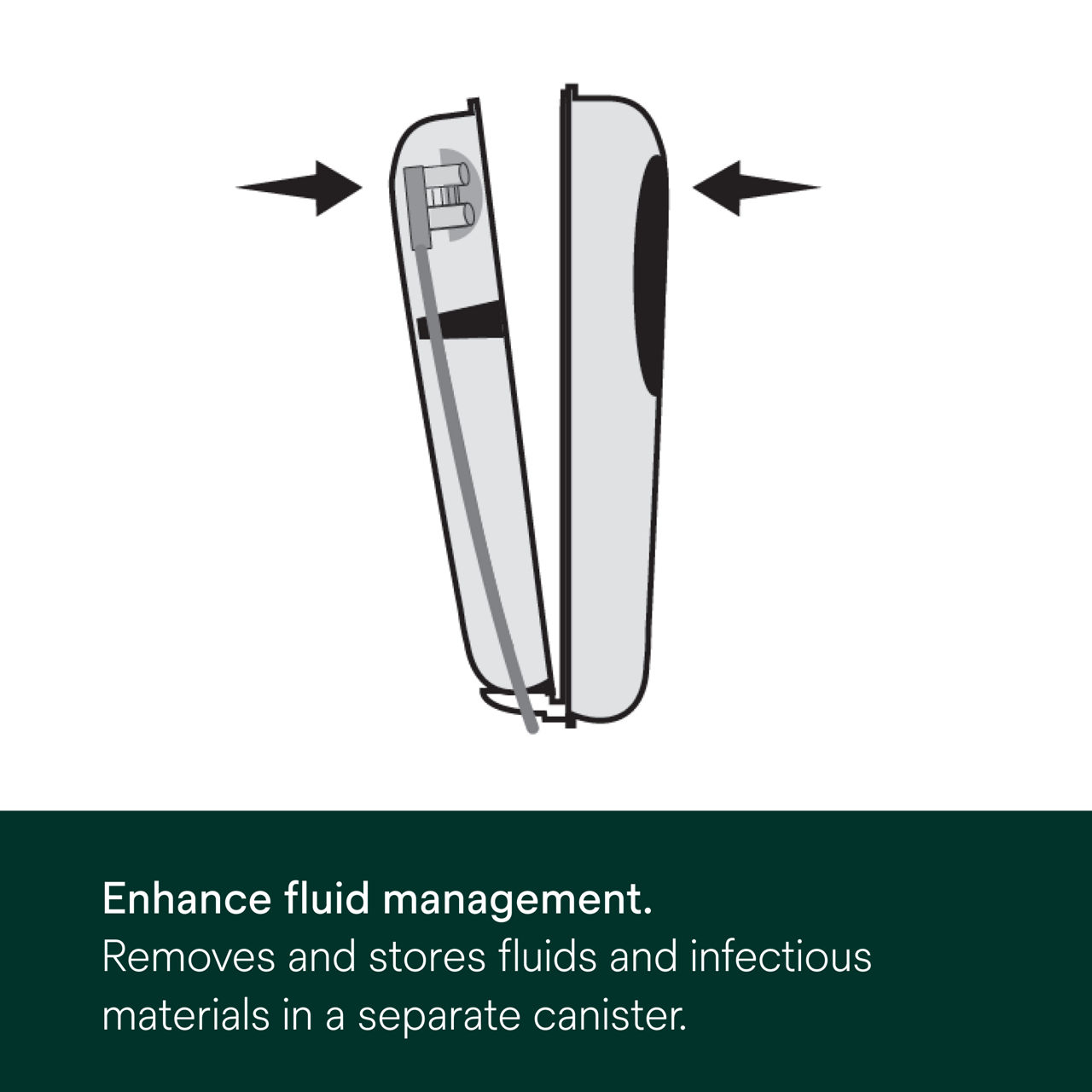
3M™ Prevena™ Plus 125 Therapy Unit (7-Day) is a portable device that promotes wounds healing, provides fluid enhancement (in a separate canister) and provides up to seven days of continuous -125mmHg NPWT.
The Prevena Plus 125 Therapy Unit is a versatile NPWT device compatible with our 3M™ Prevena™ Dressings, and 3M™ Prevena Restor™ Dressings to help promote wound healing to open wound and closed incision for patients at risk for complications.
3M™ Prevena™ Plus 125 Therapy units come with a 3M™ Prevena™ Plus 150 mL canister, a 3M™ Prevena™ Plus 125 Therapy Unit power supply and cord and a patient-friendly carrying case with an adjustable strap.
- Provides up to seven days of continuous NPWT at -125mmHg with the simple push of a button. *
- * Prevena™ Plus 125 Therapy Unit can be continuously applied for up to 14 days, but V.AC.® Dressings should be changed by a medical professional every 48–72 hours, but no less than 3 times per week
- Visual and audible safety alerts notify you and your patients of a low battery, blockage alerts or air leaks.
- Designed with our proprietary 3M™ SensaT.R.A.C.™ Pad Technology to deliver continuous and accurate NPWT to the target site.
- Includes a rechargeable battery — no need for manual battery changes.
- Attach the 3M™ Prevena™ Plus Canister (150ml) to the therapy unit to store fluid and exudate away from the surgical site.
- Use the 3M™ Prevena™ Plus 125 Therapy Unit (7-Day) with 3M™ Prevena™ Dressings, 3M™ Prevena Restor™ Dressings and select 3M™ V.A.C.® Dressings.*
- *Prevena Plus 125 Therapy Unit is compatible with small- and medium-sized V.AC.®Dressings or 3M™ V.A.C. Whitefoam™ Dressings
Suggested Applications
- Designed for closed incisions and small-to-medium open wounds.
- Use with select 3M NPWT dressings.
Legal
- 1Wilkes RP, Kilpadi DV, Zhao Y, Kazala R, McNulty A. Closed incision management with negative pressure wound therapy (CIM): biomechanics. Surg Innov 2012;19:67-75
- 2Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regen 2011;19:588-596.
Product specifications
| Brand |
Brand
Prevena™ |
| Category Name |
Category Name
NPT Devices |