3M™ Tegaderm™ CHG Chlorhexidine Gluconate Gel Pad, 1664, 1.94 in x 2.44in, 25/CAR, 4 CAR/CS, 100/CS
About the product
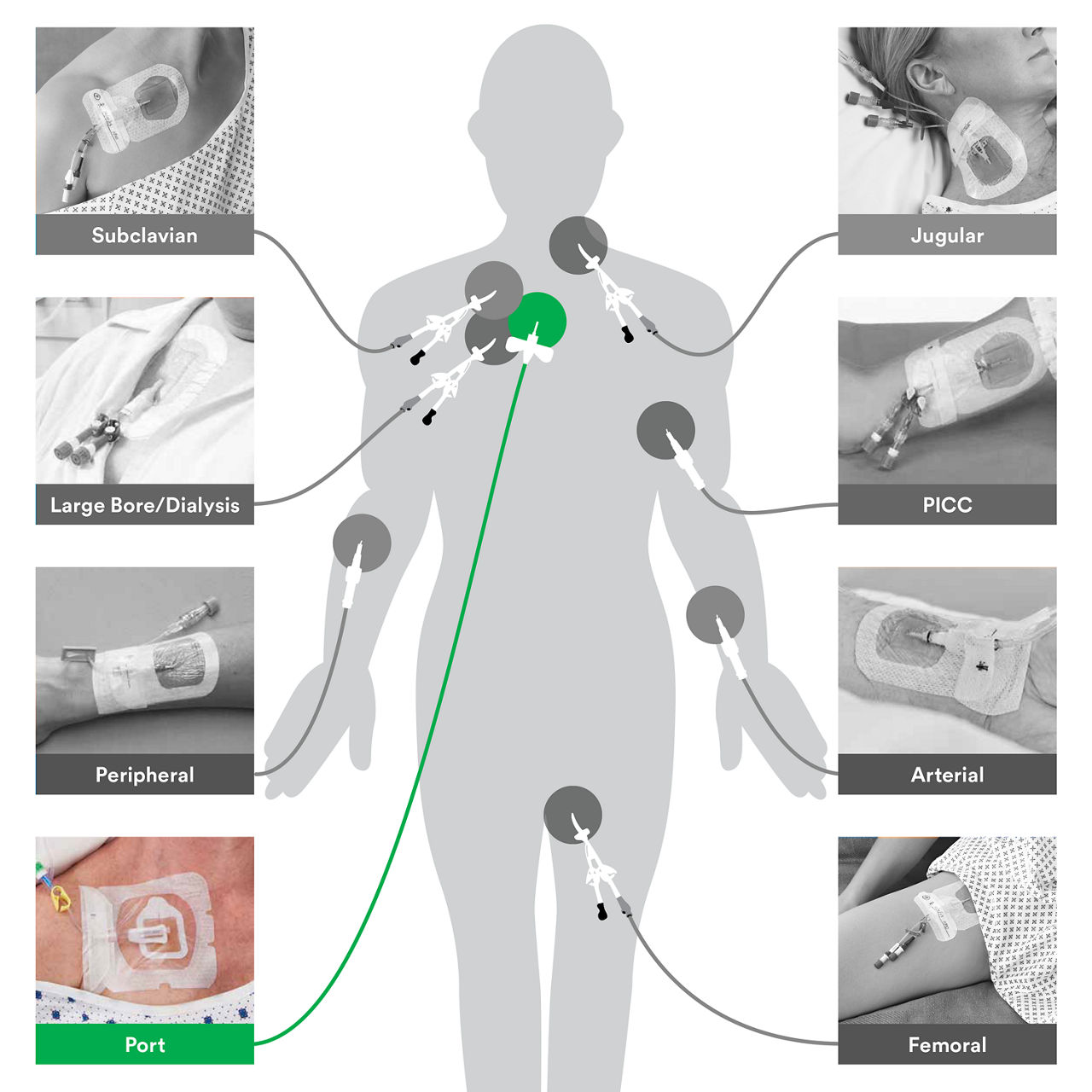
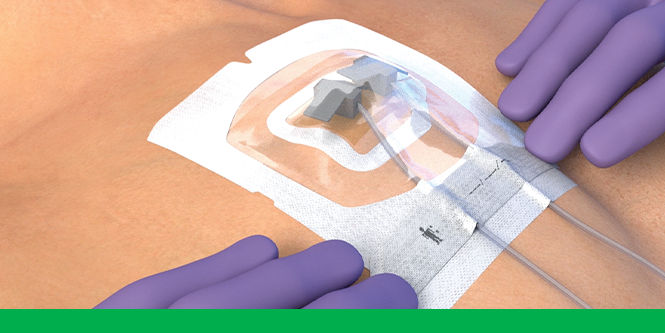
The 3M™ Tegaderm™ CHG Chlorhexidine Gluconate Gel Pad 1664 is an antimicrobial (CHG) gel pad designed to protect insertion sites and conform around a wide variety of percutaneous devices. This transparent CHG gel pad combines versatility with site visibility and antimicrobial protection.
Product Details
3M™ Tegaderm™ CHG Gel Pad 1664 provides site visibility and immediate and continuous antimicrobial activity – reducing skin flora and preventing its regrowth – for up to 7 days without additional moisture required to activate. The highly breathable film atop the CHG gel pad helps to release moisture. The gel pad remains clear and protects even in the presence of blood, saline and exudates. The adhesive border provides gentle, yet strong adherence to skin, and the pre-cut slit in the gel pad is designed to conform around a variety of percutaneous devices.
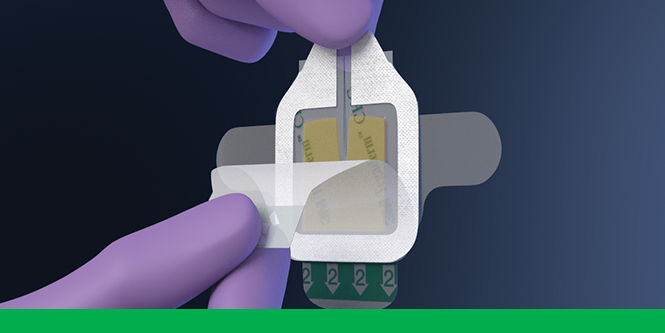
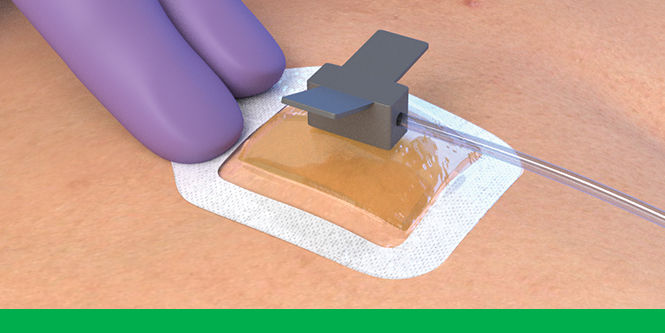
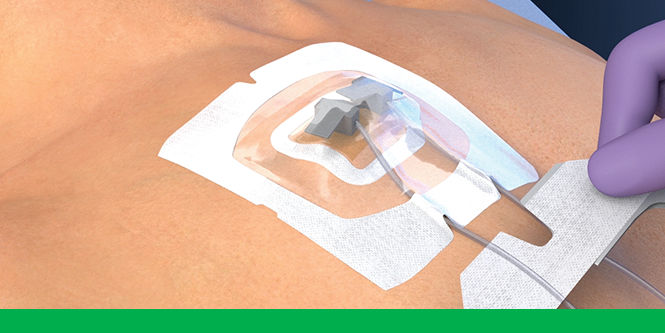
To use 3M™ Tegaderm™ CHG Chlorhexidine Gluconate Gel Pad 1664 with intravascular devices, reference the below steps.
- Standalone CHG gel pad engineered for versatility and antimicrobial protection
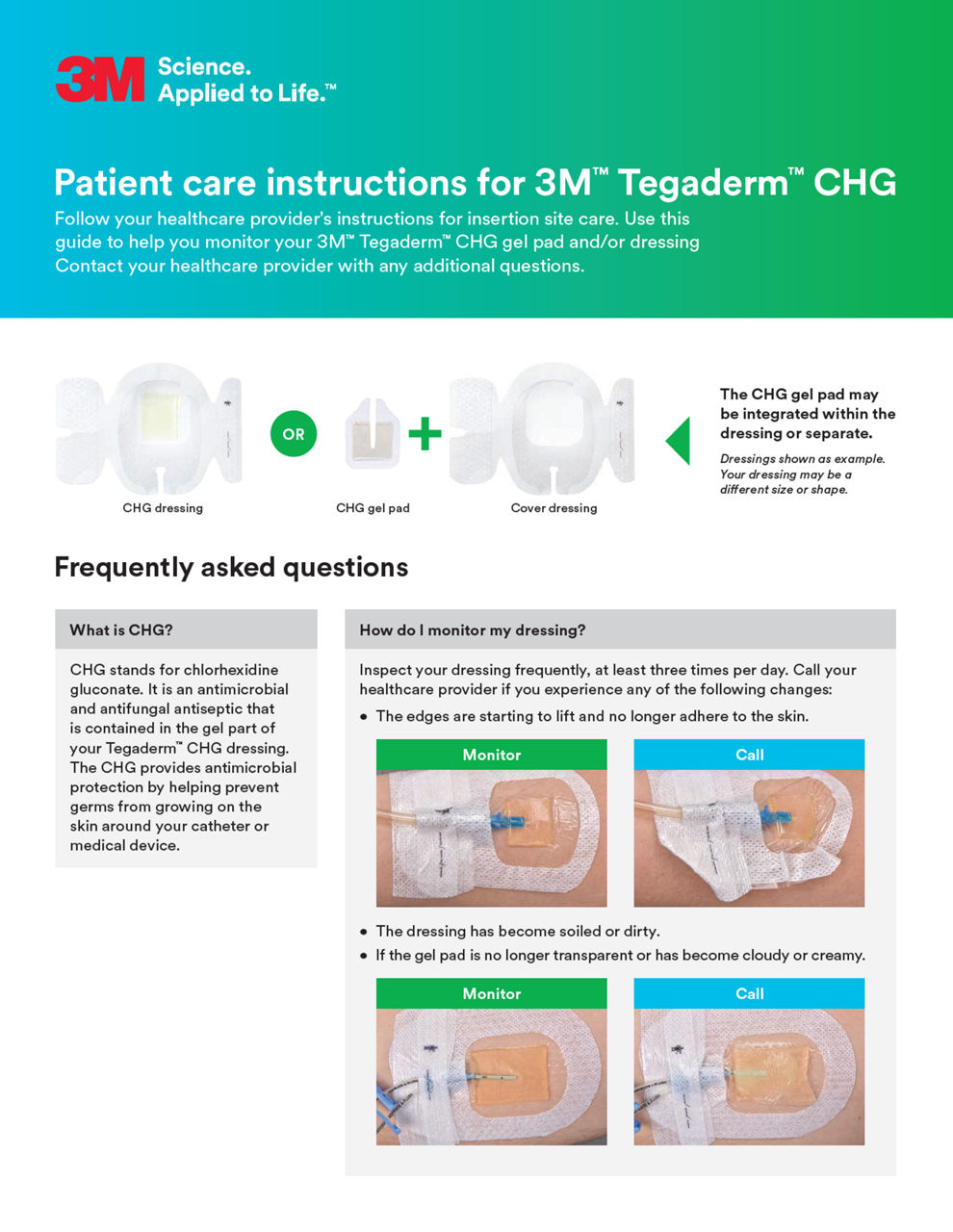
- Tegaderm™ CHG is intended to reduce catheter-related bloodstream infections (CRBSIs) in patients with central venous or arterial catheters
- CHG gel pad provides immediate and continuous antimicrobial activity – reducing skin flora and preventing its re-growth – for up to 7 days
- Pre-cut slit conforms around drains, external fixator pins, implanted venous ports and a wide variety of other percutaneous devices (up to 40 FR)
- Transparent CHG gel pad allows continuous site visibility to easily assess for early signs of infection
- Adhesive border ensures gentle, yet strong adherence to skin
- Absorbs low amount of exudate* *Bench testing shows that CHG gel can absorb 8x its weight in saline and 3x its weight in blood
- Looking for port protection and securement for patients with implanted venous ports? View the 3M™ Tegaderm™ CHG Chlorhexidine Gluconate I.V. Port Dressing, 1665
Legal
* Bench testing shows that CHG gel can absorb 8x its weight in saline and 3x its weight in bloodProduct Specifications
| Brand |
Brand
Tegaderm™ |
| Overall Length (Imperial) |
Overall Length (Imperial)
2.4400 |
| Overall Width (Metric) |
Overall Width (Metric)
4.9000 |
| Overall Length (Metric) |
Overall Length (Metric)
6.2000 |
| Dressing Type |
Dressing Type
Film |
| Overall Width (Imperial) |
Overall Width (Imperial)
1.9300 |