3M V.A.C. Therapy
Unlock the power of V.A.C.® Therapy
Solventum delivers tools to support clinical decision-making around negative pressure solutions so you can act with confidence, providing the right care at the right time to your patients. Unlock the power of VAC® Therapy with a decision guide to help you select the right NPWT solution based on the wound goals for your patient.

Proven results of V.A.C.® Therapy
There are over 2,000 peer-reviewed studies4 which have evaluated the effectiveness of V.A.C.® Therapy in a variety of settings and indicated wound types. Comparative studies have shown that V.A.C.® Therapy has been associated with:
- Fewer hospitalizations2,5
- Fewer complications5,6
- Fewer amputations7,8
- Fewer dressing changes9,10
- Faster time to wound healing11
- Shorter hospitalization7,8
- Reduced treatment times12,13
By reducing the factors that contribute to direct and indirect wound care costs, V.A.C.® Therapy has emerged as a cost-effective option for wound management.
V.A.C.® Therapy: How-to videos
Explore our video series covering all aspects of the V.A.C.® Therapy, including an overview about V.A.C.® Therapy, tips for applying V.A.C.® Dressings using the "mushroom" technique on small wounds, safety information and more.
3M™ ActiV.A.C.™ Therapy System Clinician Instructional Video
Therapy unit options to deliver V.A.C.® Therapy
Choose from a wide variety of therapy units that meet the clinical and outcome goals of different patients and different types of wounds.
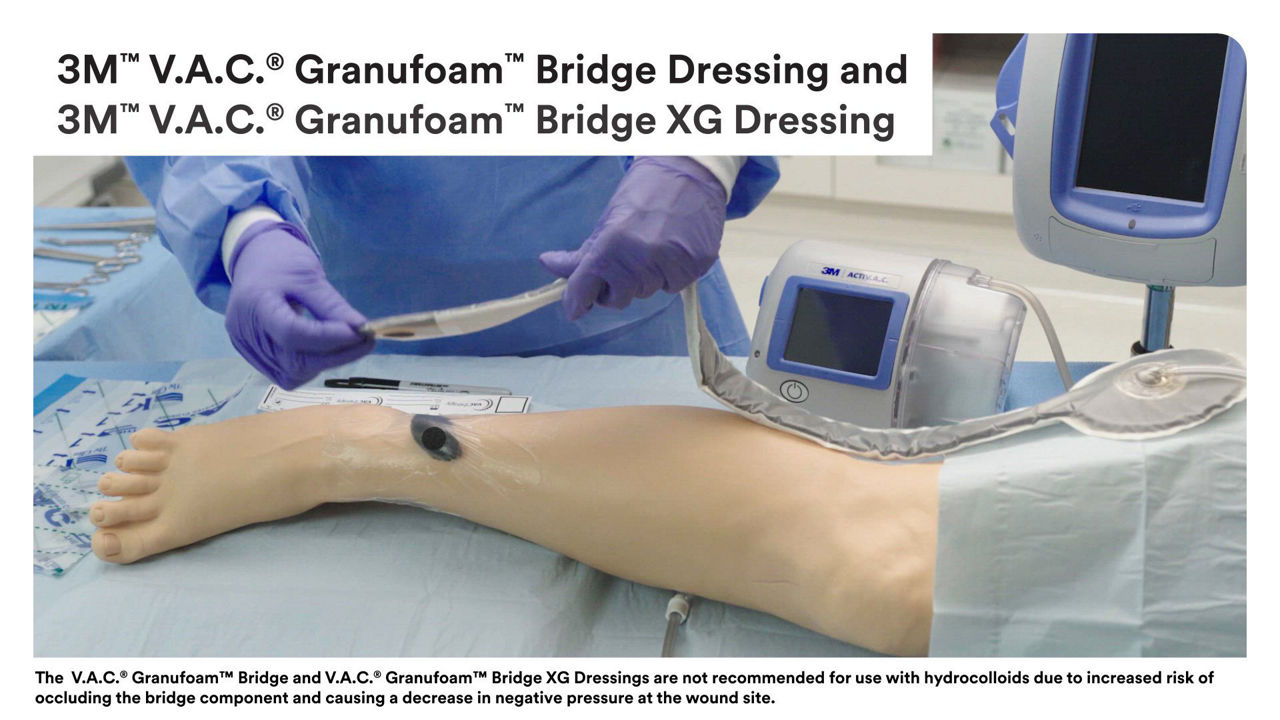
3M V.A.C.® Dressings
From dressings to kits, we have NPWT solutions to promote wound healing.

Resources
Explore more
References:
- 3M. Cumulative NPWT Wounds. 2018.
- Page JC, Newsander B, Schwenke DC, Hansen M, Ferguson J. Retrospective analysis of negative pressure wound therapy in open foot wounds with significant soft tissue defects. Adv Skin Wound Care. 2004;17(7):354-364
- Law A L. Krebs B. Karnik B. Griffin L. Comparison of Healthcare Costs Associated With Patients Receiving Traditional Negative Pressure Wound Therapies in the Post Acute Setting. Cureus 12(11): e11790.
- KCI. Percentage of V.A.C. Therapy Articles vs. Comp Articles. May 7, 2020.
- Scherer, L.A.; Shiver, S.; Chang, M.; Meredith, J.W.; Owings, J.T. The vacuum assisted closure device: a method of securing skin grafts and improving graft survival. Arch. Surg. 2002; 137:930-934.
- Falagas, M.E.; Tansarli, G.S.; Kapaskelis, A.; Vardakas, K.Z. Impact of vacuum-assisted closure (VAC) therapy on clinical outcomes of patients with sternal wound infections: a meta-analysis of non-randomized studies. PLoS One. 2013 May 31; 8(5):e64741.
- Blume, P.A.; Walters, J.; Payne, W.; Ayala, J.; Lantis, J. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care. 2008; 31:631-636.
- Armstrong, D.G.; Lavery, L.A. Diabetic Foot Study Consortium. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704-1710.
- Monsen, C.; Acosta, S.; Mani, K.; Wann-Hansson, C. A randomised study of NPWT closure versus alginate dressings in peri-vascular groin infections: quality of life, pain and cost. J. Wound Care. 2015; 24:252-260.
- Ozturk, E.; Ozguc, H.; Yilmazlar, T. The use of vacuum assisted closure therapy in the management of Fournier's gangrene. Am. J. Surg. 2009; 197:660-665.
- Yao, M.; Fabbi, M.; Hayashi, H.; et al. A retrospective cohort study evaluating efficacy in high-risk patients with chronic lower extremity ulcers treated with negative pressure wound therapy. International Wound Journal. 2014; 11:483-488.
- Sinha, K.; Chauhan, V.D.; Maheshwari, R.; Chauhan, N.; Rajan, M.; Agrawal, A. Vacuum assisted closure therapy versus standard wound therapy for open musculoskeletal injuries. Adv Orthop. 2013; 2013:245940.
- Dalla, Paola, L.; Carone, A.; Ricci, S.; Russo, A.; Ceccacci, T.; Ninkovic, S. Use of vacuum assisted closure therapy in the treatment of diabetic foot wounds. Journal of Diabetic Foot Complications. 2010; 2:33-44.
- Orgill DP, Manders EK, Sumpio BE, et al. The mechanisms of action of vacuum assisted closure: more to learn. Surgery. 2009 Jul;146(1):40-51.
- Saxena V, Hwang CW, Huang S, Eichbaum Q, Ingber D, Orgill DP. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004 Oct;114(5):1086-96; discussion 1097-8.
- McNulty AK, et al. Effects of negative pressure wound therapy on the fibroblast viability, chemotactic signaling and proliferation in a provisional wound (fibrin) matrix. WOUNDS. 2007; 15:838-‐846.
- SAT-MTF-05-982101 Evaluation of VAC Peel and Place Dressing Concepts in Full-Thickness Excisional Wounds. 2023. Allen D, Robinson T, Schmidt M, Kieswetter K. Preclinical assessment of novel longer-duration wear negative pressure wound therapy dressing in a porcine model. Wound Rep Reg. 2023;31:349-359.